Abstract
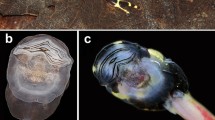
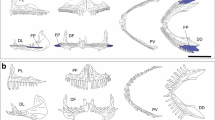
The ecomorphological guild “gastromyzophorous” joins tadpoles that inhabit flowing water and have an abdominal sucker which is employed to adhere to substrates. Historically, gastromyzophorous larvae were known in the Bufonidae and Ranidae, but a new sucker-bearing hylid tadpole was recently described from phytotelmons in Brazilian forests. We describe the larval internal anatomy of Phyllodytes gyrinaethes and ask whether its exceptional external morphology is accompanied by derived anatomical internal features that can be related to the special habitat. We also compare it to the anatomy of sucker-bearing tadpoles from other families with a focus on characters exclusive of each lineage and the shared, convergent features. The skeleton of P. gyrinaethes is highly modified relative to that of pond-type hylines and shows a profound restructuring of the oral region, palatoquadrates, and the branchial baskets. Among the muscles, besides the overall reduction in the branchial musculature, the most unusual feature in this species are the enormous, anteriorly oriented mm. levatores mandibulae externus profundus that likely produce the abduction of the two halves of the snout. The presence of the abdominal sucker is coupled with changes in some muscle trajectories and hypertrophy of the subhyoid ligaments, and the sucker connectivity differs in some aspects compared with those of bufonids and ranids (e.g., the presence of massive mm. diaphragmatopraecordialis parallel to the sucker plane). P. gyrinaethes tadpoles, with their combination of both rare and unique morphological features plus their confined microhabitat with exceptional functional and ecological requirements, represent an extreme morphotype within Hylidae and anuran tadpoles in general.







Similar content being viewed by others
Abbreviations
- Lev.:
-
Levator
- m.:
-
Musculus
- mand.:
-
Mandibulae
References
Aguayo R, Lavilla EO, Vera Candioti MF, Camacho T (2009) Living in fast-water: morphology of the gastromyzophorous tadpole of the bufonid Rhinella quechua (R. veraguensis group). J Morphol 270:1431–1442
Altig R (2006) Discussions of the origin and evolution of the oral apparatus of anuran tadpoles. Acta Herpetol 2:95–105
Altig R, McDiarmid RW (1999) Body plan: development and morphology. In: McDiarmid RW, Altig R (eds) Tadpoles: the biology of anuran larvae. University of Chicago Press, Chicago, pp 24–51
Böck P (1989) Romeis Mikroskopische Technik. Urban & Schwarzenberg, München
Böck JW, Shear CR (1972) A staining method for gross dissection of vertebrate muscles. Anat Anz 130:222–227
Carr KM, Altig R (1991) Oral disc muscles of anuran tadpoles. J Morphol 208:271–277
Dietrich HF, Fontaine AR (1975) A decalcification method for ultrastructure of echinoderm tissues. Stain Technol 50:351–354
Faivovich J, Haddad CFB, Garcia PCA, Frost DR, Campbell JA, Wheeler WC (2005) Systematic review of the frog family Hylidae, with special reference to Hylinae: phylogenetic analysis and taxonomic revision. Bull Am Mus Nat Hist 294:1–240
Gan LL, Hertwig ST, Das I, Haas A (2015) The anatomy and structural connectivity of the abdominal sucker in the tadpoles of Huia cavitympanum, with comparisons to Meristogenys jerboa (Lissamphibia: Anura: Ranidae). J Zool Syst Evol Res 54:46–59
Gosner KL (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16:183–190
Haad MB, Vera Candioti F, Baldo D (2014) The stream tadpoles of Rhinella rumbolli (Anura: Bufonidae). Herpetologica 70:184–197
Haas A (2001) Mandibular arches musculature of anuran tadpoles, with comments on homologies of amphibian jaw muscles. J Morphol 247:1–33
Haas A (2003) Phylogeny of frogs as inferred from primarily larval characters (Amphibia: Anura). Cladistics 19:23–89
Haas A, Fischer MS (1997) Three dimensional reconstruction of histological sections using modern product-design software. The Anat Rec 249:510–516
Haas A, Hertwig S, Das I (2006) Extreme tadpoles: the morphology of the fossorial megophryid larva, Leptobrachella mjobergi. Zoology 109:26–42
Haas A, Pohlmeyer J, McLeod DS, Kleinteich T, Hertwig ST, Das I, Buchholz D (2014) Extreme tadpoles II: the highly derived larval anatomy of Occidozyga baluensis (Boulenger, 1896), an obligate carnivorous tadpole. Zoomorphology 133:321–342
Handrigan GR, Haas A, Wassersug RJ (2007) Bony-tailed tadpoles: the development of supernumerary caudal vertebrae in larval megophryids (Anura). Evol Dev 9:190–202
Inger RF (1966) The systematics and zoogeography of the Amphibia of Borneo. Fieldiana Zool 52:1–402
Inger RF (1985) Tadpoles of the forested regions of Borneo. Fieldiana Zool 26:1–89
Kaplan M (1997) Internal and external anatomy of the abdominal disc of Atelopus (Bufonidae) larvae. Caldasia 19:61–69
Lannoo MJ, Townsend DS, Wassersug RJ (1987) Larval life in the leaves: arboreal tadpole types, with special attention to the morphology, ecology and behavior of the oophagous Osteopilus brunneus (Hylidae) larva. Fieldiana Zool 38:1–31
Lavilla EO, de Sá RO (2001) Chondrocranium and visceral skeleton of Atelopus tricolor and Atelophryniscus chrysophorus tadpoles (Anura. Bufonidae). Amphibia-Reptilia 22:167–177
Lehtinen RM, Lannoo MJ, Wassersug RJ (2004) Phytotelm-breeding anurans: past, present and future research. Misc Publ Mus Zool Univ Mich 193:1–9
Magalhães FM, Juncá FA, Garda AA (2015) Tadpole and vocalizations of Phyllodytes wuchereri (Anura: Hylidae) from Bahia, Brazil. Salamandra 51:83–90
Noble GK (1929) The adaptive modifications of the arboreal tadpoles of Hoplophryne and the torrent tadpoles of Staurois. Bull Am Mus Nat Hist 58:291–337
Orton GI (1953) The systematics of vertebrate larvae. Syst Zool 2:63–75
Peixoto OL, Caramaschi U, Freire EMX (2003) Two new species of Phyllodytes (Anura: Hylidae) from the state of Alagoas, northeastern Brazil. Herpetologica 59:235–246
Pusey HK (1943) On the head of the liopelmid frog, Ascaphus truei. I. The chondrocranium, jaws, arches, and muscles of a partly-grown larva. Q J Microsc Sci 84:105–185
Raj P, Vasudevan K, Singh S, Aggarwal RK (2012) Larval morphology and ontogeny of Nasikabatrachus sahyadrensis Biju & Bossuyt, 2003 (Anura, Nasikabatrachidae) from Western Ghats, India. Zootaxa 3510:65–76
Ramaswami LS (1943) An account of the chondrocranium of Rana afghana and Megophrys, with a description of the masticatory musculature of some tadpoles. Proc Natl Inst Sci India 9:43–58
Roelants K, Haas A, Bossuyt F (2011) Anuran radiations and the evolution of tadpole morphospace. PNAS 108:8731–8736
Rowley JJL, Tran DAT, Le DTT, Hoang HD, Altig R (2012) The strangest tadpole: the oophagous, tree-hole dwelling tadpole of Rhacophorus vampyrus (Anura: Rhacophoridae) from Vietnam. J Nat Hist 46:2969–2978
Sokol OM (1962) The tadpole of Hymenochirus boettgeri. Copeia 1962:272–284
Taylor W, van Dyke GC (1985) Revised procedures for staining and clearing small fishes and other vertebrates for bone and cartilage study. Cybium 9:107–119
Vera Candioti MF (2004) Morphology of premetamorphic larvae of Lysapsus limellus (Anura: Pseudinae) from Santa Fe, Argentina. Amphibia-Reptilia 25:41–54
Vera Candioti MF (2007) Anatomy of anuran tadpoles from lentic water bodies: systematic relevance and correlation with feeding habits. Zootaxa 1600:1–175
Vera Candioti MF, Haas A (2004) Three dimensional reconstruction of the hyobranchial apparatus of Hyla nana tadpoles (Anura: Hylidae). Cuad Herpetol 18:3–15
Wassersug RJ, Hoff K (1979) A comparative study of the buccal pumping mechanism of tadpoles. Biol J Linn Soc 12:225–259
Zachariah A, Abraham RK, Das S, Altig R (2012) A detailed account of the reproductive strategy and developmental stages of Nasikabatrachus sahyadrensis (Anura: Nasikabatrachidae), the only extant member of an archaic frog lineage. Zootaxa 3510:53–64
Acknowledgements
This work was supported by Consejo Nacional de Investigaciones Científicas y Técnicas, Agencia Nacional de Promoción Científica y Tecnológica, and Universidad Nacional de Tucumán Grants: PICTs 2012/2687, 2013/0404, 2014/1930, PIP 0875, and CIUNT-G430. We deeply thank E. Gretscher for serial sectioning and F. Pucci Alcaide for the photographs of histological sections. Also, we deeply thank F. Nascimento and B. Lisboa for the tadpole video recording and discussions about larvae behavior in the field.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (MP4 60918 kb)
Rights and permissions
About this article
Cite this article
Vera Candioti, F., Haas, A., Altig, R. et al. Cranial anatomy of the amazing bromeliad tadpoles of Phyllodytes gyrinaethes (Hylidae: Lophyohylini), with comments about other gastromyzophorous larvae. Zoomorphology 136, 61–73 (2017). https://doi.org/10.1007/s00435-016-0334-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-016-0334-7