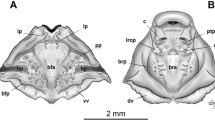
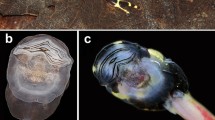
Abstract
Tadpoles of Occidozyga species have been reported to be carnivorous, feeding on insects and other tadpoles. We present photographic evidence for the previously undocumented larval feeding behavior in O. baluensis. Furthermore, we present a detailed anatomical description of the skull, cranial musculature, and gross gut morphology based on three-dimensional reconstructions from serial sections and μCT imagery. The cranial anatomy of larval O. baluensis is highly derived in many characters, with respect to taxa outside the genus Occidozyga, most notably the palatoquadrate and hyobranchial apparatus, that play a major role in tadpole feeding. A large larval stomach was present in the specimens examined, indicative of a macrophagous carnivorous mode of feeding. Because of the relatively small oral orifice, relatively large-sized food items found in the larval stomach, and the tunnel-like arrangement of structures that form the buccal cavity, we hypothesize that suction feeding utilizing strong negative pressure is employed by this species. Furthermore, we propose that force, rather than speed, is the main characteristic of their feeding. The unique features of the study species substantially expand the known morphospace for tadpoles, particularly among the Acosmanura (Pelobatoidea, Pelodytoidea, and Neobatrachia). Except for Microhylidae, acosmanurans previously described possess limited innovative larval morphologies. Larval carnivory has evolved convergently several times in distant anuran clades and shows structural, behavioral, and functional differences in the known examples.









Similar content being viewed by others
Abbreviations
- 3D:
-
Three-dimensional
- cart.:
-
Cartilago
- for.:
-
Foramen
- lev.:
-
Levator
- m.:
-
Musculus
- mand.:
-
Mandibulae
- proc.:
-
Processus
- prof.:
-
Profundus
References
Ablan D (2008) Official Luxology modo 301 guide. Course Technology, Cengage Learning, Boston
Alcala AC (1962) Breeding behavior and early development of frogs of Negros, Philippine Islands. Copeia 1962:679–726
Altig R, Johnston GF (1989) Guilds of anuran larvae: relationships among developmental modes, morphologies, and habitats. Herpetol Monogr 3:81–109
Altig R, McDiarmid RW (1999) Diversity: familial and generic characterizations. In: McDiarmid RW, Altig R (eds) Tadpoles: the biology of anuran larvae. University of Chicago Press, Chicago, pp 295–335
Altig R, Whiles M, Taylor C (2007) What do tadpoles really eat? Assessing the trophic status of an understudied and imperiled group of consumers in freshwater habitats. Freshw Biol 52:386–395
Bloom S, Ledon-Rettig C, Infante C, Everly A, Hanken J, Nascone-Yoder N (2013) Developmental origins of a novel gut morphology in frogs. Evol Dev 15:213–223
Boulenger GA (1896) Descriptions of new batrachians in the British Museum. Ann Mag Nat Hist 6:401–406
Bragg AN (1956) Dimorphism and cannibalism in tadpoles of Scaphiopus bombifrons (Amphibia, Salientia). Southwest Nat 1:105–108
Bragg AN (1964) Further study of predation and cannibalism in spadefoot tadpoles. Herpetologica 20:17–24
Brower AVZ, Schawaraoch V (1996) Three steps of homology assessment. Cladistics 12:265–272
Cannatella D (1999) Architecture: cranial and axial musculoskeleton. In: McDiarmid RW, Altig R (eds) Tadpoles: the biology of anuran larvae. University of Chicago Press, Chicago
Carroll EJ, Senevirantne AM, Ruibal R (1991) Gastric pepsin in an anuran larva. Dev Growth Differ 33:499–507
Cei JM (1968) Notes on the tadpoles and the breeding ecology of Lepidobatrachus (Amphibia: Ceratophrynidae). Herpetologica 24:141–146
Crump ML (1992) Cannibalism in amphibians. In: Elgar MA, Crespi BJ (eds) Cannibalism: ecology and evolution among diverse taxa. Oxford University Press, Oxford, pp 256–276
Das I (1995) Comparative morphology of the gastrointestinal tract in relation to diet in frogs from a locality in south India. Amphibia-Reptilia 16:289–293
De Beer GR (1937) The development of the vertebrate skull. The University of Chicago Press, Chicago
De Jongh HJ (1968) Functional morphology of the jaw apparatus of larval and metamorphosing Rana temporaria. Neth J Zool 18:1–103
De Jongh HJ, Gans C (1969) On the mechanism of respiration in the bullfrog, Rana catesbeiana. J Morphol 127:259–290
De Pinna MCC (1991) Concepts and tests of homology in the cladistic paradigm. Cladistics 7:367–394
Deban SM, Olson WM (2002) Suction feeding by a tiny predatory tadpole. Nature 420:41–42
Dietrich HF, Fontaine AR (1975) A decalcification method for ultrastructure of echinoderm tissues. Stain Technol 50:351–354
Dingerkus G, Uhler LD (1977) Enzyme clearing of Alcian Blue stained whole small vertebrates for demonstration of cartilage. Stain Technol 52:229–232
Dodd JM (1950) Ciliary feeding mechanism in anuran larvae. Nature 165:283
Drewes RC, Altig R, Howell KM (1989) Tadpoles of three frog species endemic to the forests of the Eastern Arc Mountains, Tanzania. Amphibia-Reptilia 10:435–443
Fabrezi M (2011) Heterochrony in growth and development in anurans from the Chaco of South America. Evol Biol 38:390–411
Fabrezi M, Lobo F (2009) Hyoid skeleton, its related muscles, and morphological novelties in the frog Lepidobatrachus (Anura, Ceratophryidae). Anat Rec 292:1700–1712
Fabrezi M, Quinzio SI (2008) Morphological evolution in Ceratophryinae frogs (Anura, Neobatrachia): the effects of heterochronic changes during larval development and metamorphosis. Zool J Linn Soc 154:752–780
Fox S (1990) Opportunistic cannibalism in tadpoles of the Great Basin Spadefoot Toad, Scaphiopus intermontanus. MA Thesis, San Francisco State University
Frost DR (2013) Amphibian Species of the World: an Online Reference. Version 5.6 (9 Jan 2013). Accessible at: http://research.amnh.org/herpetology/amphibia/. American Museum of Natural History, New York
Frost DR, Grant T, Faivovich J, Bain RH, Haas A, Haddad CFB, De Sá RO, Channing A, Wilkinson M, Donnellan SC, Raxworthy CJ, Campbell JA, Blotto BL, Moler P, Drewes RC, Nussbaum RA, Lynch JD, Green DM, Wheeler WC (2006) The amphibian tree of life. Bull Am Mus Nat Hist 297:1–370
Fry AE, Kaltenbach JC (1992) Gastrointestinal tract length in two species of anuran tadpoles, Ceratophrys ornata and Rana pipiens. Am Zool 32:23A
Fry AE, Kaltenbach JC (1999) Histology and lectin-binding patterns in the digestive tract of the carnivorous larvae of the anuran Ceratophrys ornata. J Morphol 241:19–32
Gaupp E (1893) Beitraege zur Morphologie des Schaedels. I. Primordial-Cranium und Kieferbogen von Rana fusca. Morphol Arb 2:275–481
Gaupp E (1894) Beitraege zur Morphologie des Schaedels. II. Das Hyo-Branchial-Skelet der Anuren und seine Umwandlung. Morphol Arb 3:389–437
Gaupp E (1896) Ecker’s und Wiedersheim’s Anatomie des Frosches. Erste Abteilung, Lehre vom Skelet und vom Muskelsystem. Friedrich Vieweg und Sohn, Braunschweig
Gosner KL (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16:183–190
Gradwell N (1972) Gill irrigation in Rana catesbeiana. Part I. On the anatomical basis. Can J Zool 50:481–499
Griffiths I (1961) The form and function of the foregut in anuran larvae (Amphibia: Salientia) with particular reference to the Manicotto Glandulare. Proc Zool Soc Lond 137:249–283
Grosjean S, Vences M, Dubois A (2004) Evolutionary significance of oral morphology in the carnivorous tadpoles of tiger frogs, genus Hoplobatrachus (Ranidae). Biol J Linn Soc 81:171–181
Haas A (1997) The larval hyobranchial apparatus of discoglossoid frogs: its structure and bearing on the systematics of the Anura (Amphibia: Anura). J Zool Syst Evol Res 53:179–197
Haas A (1999) Larval and metamorphic skeletal development in the fast developing frog Pyxicephalus adspersus (Anura, Ranidae). Zoomorphology 119:23–35
Haas A (2001) Mandibular arch musculature of anuran tadpoles, with comments on homologies of amphibian jaw muscles. J Morphol 247:1–33
Haas A (2003) Phylogeny of frogs as inferred from primarily larval characters (Amphibia: Anura). Cladistics 19:23–89
Haas A, Hertwig S, Das I (2006) Extreme tadpoles: the morphology of the fossorial megophryid larva, Leptobrachella mjobergi. Zoology 109:26–42
Haas A, Richards SJ (1998) Correlations of cranial morphology, ecology, and evolution in Australian suctorial tadpoles of the genera Litoria and Nyctimystes (Amphibia: Anura: Hylidae: Pelodryadinae). J Morphol 238:109–141
Harris RN (1999) The anuran tadpole: evolution and maintenance. In: McDiarmid RW, Altig R (eds) Tadpoles: the biology of anuran larvae. The University of Chicago Press, Chicago, IL, pp 279–294
Heyer WR (1973) Ecological interaction of frog larvae at a seasonal tropical location in Thailand. J Herpetol 7:337–361
Hintze-Podufal C, Schroer H (1989) Aspects of Hymenochirus boettgeri development. Fortschr Zool 35:283–286
Inger RF (1985) Tadpoles of the forested regions of Borneo. Fieldiana Zool New Ser 26:1–89
Inger RF, Stuebing RB (2005) A field guide to the frogs of Borneo. Natural History Publications (Borneo), Sdn Bhd, Kota Kinabalu
Ishizuya-Oka A, Shimozawa A (1987) Development of the connective tissue in the digestive tract of the larval and metamorphosing Xenopus laevis. Anat Anz Jena 164:81–93
Iskandar DT (1998) The amphibians of Java and Bali. Bogor
Iskandar DT, Arifin U, Rachmansah A (2011) A new frog (Anura, Dicroglossidae) related to Occidozyga semipalmata Smith, 1927, from the eastern Peninsula of Sulawesi, Indonesia. Raffles Bull Zool 59:219–228
Jungfer K-H (1996) Reproduction and parental care of the coronated treefrog, Anotheca spinosa (Steindachner, 1864) (Anura: Hylidae). Herpetologica 52:25–32
Kaltenbach JC, Fry AE, Colpitts KM, Faszewski EE (2012) Apoptosis in the digestive tract of herbivorous Rana pipiens larvae and carnivorous Ceratophrys ornata larvae: an immunohistochemical study. J Morphol 273:103–108
Kenny JS (1969) Feeding mechanisms in anuran larvae. J Zool Lond 157:225–246
Kleinteich T, Haas A (2006) Cranial musculature in the larva of the caecilian, Ichthyophis kohtaoensis (Lissamphibia: Gymnophiona). J Morphol 268:74–88
Kleinteich T, Haas A (2011) The hyal and ventral branchial muscles in caecilian and salamander larvae: homologies and evolution. J Morphol 272:598–613
Lambertini G (1929) Il manicotto glandulare di Rana esculenta nei suoi aspetti strutturali e nelle sue evoluzioni metamorfiche durante lo sviluppo. Ric Morfol Roma 9:71–88
Lavilla EO (1990) The tadpole of Hyla nana (Anura: Hylidae). J Herpetol 24:207–209
Lavilla EO, de Sá R (2001) Chondrocranium and visceral skeleton of Atelopus tricolor and Atelophryniscus chrysophorus tadpoles (Anura, Bufonidae). Amphibia-Reptilia 22:167–177
Lavilla EO, Fabrezi M (1992) Anatomia craneal de larvas de Lepidobatrachus llanensis y Ceratophrys cranwelli (Anura: Leptodactylidae). Acta Zool Lilloana 42:5–11
Leong TM, Chou LM (1999) Larval diversity and development in the Singapore Anura (Amphibia). Raffles Bull Zool 47:81–137
Malkmus R, Manthey U, Vogel G, Hoffmann P, Kosuch J (2002) Amphibians and Reptiles of Mount Kinabalu (North Borneo). A.R.G. Gantner K.G., Koeltz Scientific Books, Koenigstein
McAvoy JW, Dixon KE (1977) Cell proliferation and renewal in the small intestine epithelium of metamorphosing and adult Xenopus laevis. J Exp Zool 202:129–138
McDiarmid RW, Altig R (1999) Tadpoles: the biology of anuran larvae. University Chicago Press, Chicago
Metscher BD (2009) MicroCT for comparative morphology: simple staining methods allow high-contrast 3D imaging of diverse non-mineralized animal tissues. BMC Physiol 9:1–14
Mulisch M, Welsch U (2010) Romeis-Mikroskopische Technik. Spektrum Akademischer Verlag, Heidelberg
Natale GS, Alcalde L, Herrera R, Cajade R, Schaefer EF, Marangoni F, Trudeau VL (2011) Underwater acoustic communication in the macrophagic carnivorous larvae of Ceratophrys ornata (Anura: Ceratophryidae). Acta Zool 92:46–53
Orton GL (1953) The systematics of vertebrate larvae. Syst Zool 2:63–75
Patterson C (1982) Morphological characters and homology. In: Joysey KA, Friday AE (eds) Problems of phylogenetic reconstruction. Academic Press, New York
Petranka JW, Kennedy CA (1999) Pond tadpoles with generalized morphology: is it time to reconsider their functional roles in aquatic communities? Oecologia 120:621–631
Pfennig DW (1992) Polyphenism in spadefoot toad tadpoles as a locally adjusted evolutionarily stable strategy. Evolution 46:1408–1420
Pomeroy, LV (1981) Developmental polymorphism in the tadpoles of the spadefoot toad Scaphiopus multiplicatus. PhD dissertation. University of California, Riverside
Pope CH (1931) Notes on amphibians from Fukien, Hainan, and other parts of China. Bull Am Mus Nat Hist 61:397–610
Púgener A, Maglia AM, Trueb L (2003) Revisiting the contribution of larval characters to an analysis of phylogenetic relationships of basal anurans. Zool J Linn Soc 139:129–155
Pusey HK (1943) On the head of the liopelmid frog, Ascaphus truei: I. The chrondrocranium, jaws, arches, and muscles of a partly-grown larva. Q J Microsc Sci 84:105–185
Pyron AR, Wiens JJ (2011) A large-scale phylogeny of Amphibia including over 2800 species, and a revised classification of extant frogs, salamanders, and caecilians. Mol Phyl Evol 61:543–583
Richter SS (2005) Homologies in phylogenetic analyses—concept and tests. Theory Biosci 124:105–120
Rieppel O, Kearney M (2002) Similarity. Biol J Linn Soc 75:59–82
Roček Z (2003) Larval development and evolutionary origin of the anuran skull. In: Heatwole H, Davies M (eds) Amphibian biology. Surrey Beatty, Sons PTY Limited, Chipping Norton, pp 1877–1995
Roelants K, Gower DJ, Wilkinson M, Loader SP, Biju SD, Guillaume K, Moriau L, Bossuyt F (2007) Global patterns of diversification in the history of modern amphibians. PNAS 104:887–892
Roelants K, Haas A, Bossuyt F (2011) Anuran radiations and the evolution of tadpole morphospace. PNAS 108:8731–8736
Ruibal R, Thomas E (1988) The obligate carnivorous larvae of the frog, Lepidobatrachus laevis (Leptodactylidae). Copeia 1988:591–604
Satel SL, Wassersug RJ (1981) On the relative sizes of buccal floor depressor and elevator musculature in tadpoles. Copeia 1981:129–137
Sheil CA (1999) Osteology and skeletal development of Pyxicephalus adspersus (Anura: Ranidae: Raninae). J Morphol 240:49–75
Shi Y-B, Ishizuya-Oka A (1996) Biphasic intestinal development in amphibians: embryogenesis and remodeling during metamorphosis. Curr Top Dev Biol 32:205–235
Smith MA (1916) On the frogs of the genus Oxyglossis. J Nat Hist Soc Siam 2:172–175
Sokol OM (1962) The tadpole of Hymenochirus boettgeri. Copeia 1962:273–284
Sokol OM (1975) The phylogeny of anuran larvae: a new look. Copeia 1975:1–23
Sokol OM (1981) The larval chondrocranium of Pelodytes punctatus, with a review of tadpole chondrocrania. J Morphol 169:161–183
Stevens CE, Hume ID (1995) Comparative physiology of the vertebrate digestive system. Cambridge University Press, Cambridge
Storz BL, Travis J (2007) Temporally dissociated, trait-specific modifications underlie phenotypic polyphenism in Spea multiplicata, which suggests modularity. Sci World J 7:715–726
Taylor EH, Elbel RE (1958) Contribution to the herpetology of Thailand. Univ Kans Sci Bull 38:1033–1189
Taylor WR, Van Dyke GC (1985) Revised procedures for staining and clearing small fishes and other vertebrates for bone and cartilage study. Cybium 9:107–119
Ueck M (1967) Der Manicotto Glandulare (“Drüsenmagen”) der Anurenlarve. Z wiss Zool 176:173–270
Ulloa Kreisel ZE (2002) Caracteriticas morfologicas del tubo digestivo en larvas carnivoras de Lepidobatrachus llanensis (Anura: Leptodactylidae). Acta Zool Lilloana 46:31–38
Veeranagoudar DK, Radder RS, Shanbhag BA, Saidapur SK (2009) Jumping behavior of semiterrestrial tadpoles of Indirana beddomii (Günth.): relative importance of tail and body size. J Herpetol 43:680–684
Vera Candioti MF (2005) Morphology and feeding in tadpoles of Ceratophrys cranwelli (Anura: Leptodactylidae). Acta Zool 86:1–11
Vera Candioti MF (2007) Anatomy of anuran tadpoles from lentic water bodies: systematic relevance and correlation with feeding habits. Zootaxa 1600:1–175
Vera Candioti MF (2008) Larval anatomy of Andean tadpoles of Telmatobius (Anura: Ceratophryidae) from northwestern Argentina. Zootaxa 1938:40–60
Vera Candioti MF, Lavilla EO, Echeverria DD (2004) Feeding mechanisms of two treefrogs, Hyla nana and Scinax nasicus (Anura: Hylidae). J Morph 261:206–224
Wassersug RJ (1980) Internal oral features of larvae from eight anuran families: functional, systematic, evolutionary, and ecological considerations. Misc Pub Mus Nat Hist Kans 68:1–146
Wassersug R (1984) The Pseudohemisus tadpole: a morphological link between microhylid (Orton type 2) and ranoid (Orton type 4) larvae. Herpetologica 40:138–149
Wassersug RJ (1989) What, if anything is a microhylid (Orton type II) tadpole? In: Splechtna H (ed) Trends in vertebrate morphology. G. Fischer, Stuttgart, pp 534–538
Wassersug R, Heyer WR (1988) A survey of internal oral features of leptodactylid larvae. Smith Contr Zool 457:1–99
Wassersug RJ, Pyburn WF (1987) The biology of the Pe-ret’ toad Otophryne robusta (Microhylidae), with special consideration of its fossorial larva and systematic relationships. Zool J Linn Soc 91:137–169
Zhang P, Liang D, Mao RL, Hillis DM, Wake DB, Cannatella DC (2013) Efficient sequencing of anuran mtDNAs and a mitogenomic exploration of the phylogeny and evolution of frogs. Mol Biol Evol 30:1899–1915
Ziermann JM, Infante C, Hanken J, Olsson L (2011) Morphology of the cranial skeleton and musculature in the obligate carnivorous tadpole of Lepidobatrachus laevis (Anura: Ceratophryidae). Acta Zool 94:101–112
Acknowledgments
We wish to thank the Sarawak Forest Department, in particular Datuk Cheong Ek Choon, Director, and Bolhan Budeng, for issuing collecting permits (NPW.907.4–36) and export permits. The Economic Planning Unit, The Prime Minister’s Department, Malaysia, and especially Mrs. Munirah Abd. Manan were supportive in issuing research permit No. 1168 to A. Haas. Sabah Parks provided research permit TS/PTD/5/4, and we thank J. Nais. At the DESY facility, work would not have been possible without unconditional support of F. Beckmann and J. Herzen. We received skillful help from A.M. Vogt with some of the serial sectioning and clearing and staining. We gratefully acknowledge funding of the Volkswagen Foundation, Germany (Grant I/79 405 to AH and ID), Naturhistorisches Museum der Burgergemeinde Bern, Universität Hamburg, and Universiti Malaysia Sarawak. The work at Gunung Mulu National Park was generously supported in many different ways and on several occasions by Brian and Sue Clark and their staff, whom we cannot thank enough. The work of Rolf Beutel and Frank Friedrich for determination of the beetle larvae extracted from O. baluensis tadpoles is much appreciated.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Schmidt-Rhaesa.
Rights and permissions
About this article
Cite this article
Haas, A., Pohlmeyer, J., McLeod, D.S. et al. Extreme tadpoles II: the highly derived larval anatomy of Occidozyga baluensis (Boulenger, 1896), an obligate carnivorous tadpole. Zoomorphology 133, 321–342 (2014). https://doi.org/10.1007/s00435-014-0226-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-014-0226-7