Abstract
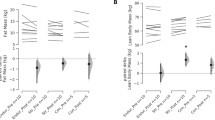
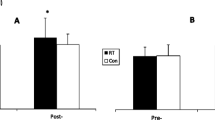
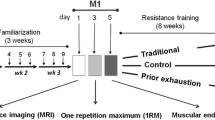
Thirty-four untrained women participated in a 6-week program to investigate slow-speed versus “normal” speed resistance-training protocols. Subjects were divided into: slow-speed (SS), normal-speed/traditional-strength (TS), normal-speed/traditional muscular endurance (TE), and non-exercising control (C) groups. Leg press, squats, and knee extensions were performed 2 days/week for the first week and 3 days/week for the remaining 5 weeks (~2 min rest). The SS group performed 6–10 repetitions maximum (6–10RM) for each set with 10 s concentric (con) and 4 s eccentric (ecc) contractions. The TS and TE groups performed sets of 6–10RM and 20–30RM, respectively, at “normal” speed (1–2 s/con and ecc contractions). TE and SS trained at the same relative intensity (~40–60% 1RM), whereas TS trained at ~80–85% 1RM. Pre- and post-training muscle biopsies were analyzed for fiber-type composition, cross-sectional area (CSA), and myosin heavy chain (MHC) content. The percentage of type IIX fibers decreased and IIAX increased in all three training groups. However, only TS showed an increase in percentage of type IIA fibers. CSA of fiber types I, IIA, and IIX increased in TS. In SS, only the CSA of IIA and IIX fibers increased. These changes were supported by MHC data. No significant changes for any parameters were found for the C group. In conclusion, slow-speed strength training induced a greater adaptive response compared to training with a similar resistance at “normal” speed. However, training with a higher intensity at “normal” speed resulted in the greatest overall muscle fiber response in each of the variables assessed.



Similar content being viewed by others
References
Bar A, Pette D (1988) Three fast myosin heavy chains in adult rat skeletal muscle. FEBS Lett 235:153–155
Bergström J (1962) Muscle electrolytes in man. Scand J Clin Lab Invest 14(Suppl 68):1–110
Blomstrand E, Ekblom B (1982) The needle biopsy technique for fibre type determination in human skeletal muscle—a methodological study. Acta Physiol Scand 116:437–442
Brooke MH, Kaiser KK (1970) Three “myosin adenosine triphosphatase” systems: the nature of their pH lability and sulfhydryl dependence. J Histochem Cytochem 18:670–672
Burd NA, West DW, Staples AW, Atherton PJ, Baker JM, Moore DR, Holwerda AM, Parise G, Rennie MJ, Baker SK, Phillips SM (2010) Low-load high volume resistance exercise stimulates muscle protein synthesis more than high-load low volume resistance exercise in young men. PLoS One 5:e12033
Campos GE, Luecke TJ, Wendeln HK, Toma K, Hagerman FC, Murray TF, Ragg KE, Ratamess NA, Kraemer WJ, Staron RS (2002) Muscular adaptations in response to three different resistance-training regimens: specificity of repetition maximum training zones. Eur J Appl Physiol 88:50–60
Claflin DR, Larkin LM, Cederna PS, Horowitz JF, Alexander NB, Cole NM, Galecki AT, Chen S, Nyquist LV, Carlson BM, Faulkner JA, Ashton-Miller JA (2011) Effects of high- and low-velocity resistance training on the contractile properties of skeletal muscle fibers from young and older humans. J Appl Physiol
Cormie P, McGuigan MR, Newton RU (2011) Developing maximal neuromuscular power: part 1—biological basis of maximal power production. Sports Med 41:17–38
Coyle EF, Feiring DC, Rotkis TC, Cote RW 3rd, Roby FB, Lee W, Wilmore JH (1981) Specificity of power improvements through slow and fast isokinetic training. J Appl Physiol Respir Environ Exerc Physiol 51:1437–1442
Deschenes MR, Kraemer WJ (2002) Performance and physiologic adaptations to resistance training. Am J Phys Med Rehabil 81:S3–S16
Ennion S, Sant’ana Pereira J, Sargeant AJ, Young A, Goldspink G (1995) Characterization of human skeletal muscle fibres according to the myosin heavy chains they express. J Muscle Res Cell Motil 16:35–43
Evans WJ, Phinney SD, Young VR (1982) Suction applied to a muscle biopsy maximizes sample size. Med Sci Sports Exerc 14:101–102
Fry AC, Allemeier CA, Staron RS (1994) Correlation between percentage fiber type area and myosin heavy chain content in human skeletal muscle. Eur J Appl Physiol Occup Physiol 68:246–251
Gillies EM, Putman CT, Bell GJ (2006) The effect of varying the time of concentric and eccentric muscle actions during resistance training on skeletal muscle adaptations in women. Eur J Appl Physiol 97:443–453
Green H, Goreham C, Ouyang J, Ball-Burnett M, Ranney D (1998) Regulation of fiber size, oxidative potential, and capillarization in human muscle by resistance exercise. Am J Physiol 276:R591–R596
Guth L, Samaha FJ (1969) Qualitative differences between actomyosin ATPase of slow and fast mammalian muscle. Exp Neurol 25:138–152
Hatfield DL, Kraemer WJ, Spiering BA, Hakkinen K, Volek JS, Shimano T, Spreuwenberg LP, Silvestre R, Vingren JL, Fragala MS, Gomez AL, Fleck SJ, Newton RU, Maresh CM (2006) The impact of velocity of movement on performance factors in resistance exercise. J Strength Cond Res 20:760–766
Hunter GR, Seelhorst D, Snyder S (2003) Comparison of metabolic and heart rate responses to super slow vs. traditional resistance training. J Strength Cond Res 17:76–81
Hutchins K (1992) Superslow: the ultimate exercise protocol, 2nd edn. Media Support, Casselberry
Jackson AS, Pollock ML, Ward A (1980) Generalized equations for predicting body density of women. Med Sci Sports Exerc 12:175–181
Keeler LK, Finkelstein LH, Miller W, Fernhall B (2001) Early-phase adaptations of traditional-speed vs. superslow resistance training on strength and aerobic capacity in sedentary individuals. J Strength Cond Res 15:309–314
Kovaleski JE, Heitman RH, Trundle TL, Gilley WF (1995) Isotonic preload versus isokinetic knee extension resistance training. Med Sci Sports Exerc 27:895–899
Kraemer WJ, Ratamess NA (2004) Fundamentals of resistance training: progression and exercise prescription. Med Sci Sports Exerc 36:674–688
Kraemer WJ, Patton JF, Gordon SE, Harman EA, Deschenes MR, Reynolds K, Newton RU, Triplett NT, Dziados JE (1995) Compatibility of high-intensity strength and endurance training on hormonal and skeletal muscle adaptations. J Appl Physiol 78:976–989
Kraemer WJ, Adams K, Cafarelli E, Dudley GA, Dooly C, Feigenbaum MS, Fleck SJ, Franklin B, Fry AC, Hoffman JR, Newton RU, Potteiger J, Stone MH, Ratamess NA, Triplett-McBride T (2002) American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc 34:364–380
Morrissey MC, Harman EA, Frykman PN, Han KH (1998) Early phase differential effects of slow and fast barbell squat training. Am J Sports Med 26:221–230
Munn J, Herbert RD, Hancock MJ, Gandevia SC (2005) Resistance training for strength: effect of number of sets and contraction speed. Med Sci Sports Exerc 37:1622–1626
Neils CM, Udermann BE, Brice GA, Winchester JB, McGuigan MR (2005) Influence of contraction velocity in untrained individuals over the initial early phase of resistance training. J Strength Cond Res 19:883–887
Perrie WT, Bumford SJ (1986) Electrophoretic separation of myosin isoenzymes. Implications for the histochemical demonstration of fibre types in biopsy specimens of human skeletal muscle. J Neurol Sci 73:89–96
Rana SR, Chleboun GS, Gilders RM, Hagerman FC, Herman JR, Hikida RS, Kushnick MR, Staron RS, Toma K (2008) Comparison of early phase adaptations for traditional strength and endurance, and low velocity resistance training programs in college-aged women. J Strength Cond Res 22:119–127
Remaud A, Cornu C, Guevel A (2009) Agonist muscle activity and antagonist muscle co-activity levels during standardized isotonic and isokinetic knee extensions. J Electromyogr Kinesiol Off J Int Soc Electrophysiol Kinesiol 19:449–458
Sakamoto A, Sinclair PJ (2006) Effect of movement velocity on the relationship between training load and the number of repetitions of bench press. J Strength Cond Res 20:523–527
Shepstone TN, Tang JE, Dallaire S, Schuenke MD, Staron RS, Phillips SM (2005) Short-term high- vs. low-velocity isokinetic lengthening training results in greater hypertrophy of the elbow flexors in young men. J Appl Physiol 98:1768–1776
Siegel JA, Gilders RM, Staron RS, Hagerman FC (2002) Human muscle power output during upper- and lower-body exercises. J Strength Cond Res Natl Strength Cond Assoc 16:173–178
Smerdu V, Karsch-Mizrachi I, Campione M, Leinwand L, Schiaffino S (1994) Type IIx myosin heavy chain transcripts are expressed in type IIb fibers of human skeletal muscle. Am J Physiol 267:C1723–C1728
Spiering BA, Kraemer WJ, Anderson JM, Armstrong LE, Nindl BC, Volek JS, Maresh CM (2008) Resistance exercise biology: manipulation of resistance exercise programme variables determines the responses of cellular and molecular signalling pathways. Sports Med 38:527–540
Staron RS (1991) Correlation between myofibrillar ATPase activity and myosin heavy chain composition in single human muscle fibers. Histochemistry 96:21–24
Staron RS, Hagerman FC, Hikida RS, Murray TF, Hostler DP, Crill MT, Ragg KE, Toma K (2000) Fiber type composition of the vastus lateralis muscle of young men and women. J Histochem Cytochem 48:623–629
Staron RS, Hikida RS (1992) Histochemical, biochemical, and ultrastructural analyses of single human muscle fibers, with special reference to the C-fiber population. J Histochem Cytochem 40:563–568
Staron RS, Karapondo DL, Kraemer WJ, Fry AC, Gordon SE, Falkel JE, Hagerman FC, Hikida RS (1994) Skeletal muscle adaptations during early phase of heavy-resistance training in men and women. J Appl Physiol 76:1247–1255
Staron RS, Leonardi MJ, Karapondo DL, Malicky ES, Falkel JE, Hagerman FC, Hikida RS (1991) Strength and skeletal muscle adaptations in heavy-resistance-trained women after detraining and retraining. J Appl Physiol 70:631–640
Staron RS, Malicky ES, Leonardi MJ, Falkel JE, Hagerman FC, Dudley GA (1990) Muscle hypertrophy and fast fiber type conversions in heavy resistance-trained women. Eur J Appl Physiol Occup Physiol 60:71–79
Tanimoto M, Ishii N (2006) Effects of low-intensity resistance exercise with slow movement and tonic force generation on muscular function in young men. J Appl Physiol 100:1150–1157
Westcott WL, Winett RA, Anderson ES, Wojcik JR, Loud RL, Cleggett E, Glover S (2001) Effects of regular and slow speed resistance training on muscle strength. J Sports Med Phys Fit 41:154–158
Acknowledgments
We wish to thank all those individuals who assisted in supervising the training, and especially to the subjects who volunteered and worked so hard throughout the study.
Conflict of interest
The authors have no conflicts of interest to report.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by William J. Kraemer .
Rights and permissions
About this article
Cite this article
Schuenke, M.D., Herman, J.R., Gliders, R.M. et al. Early-phase muscular adaptations in response to slow-speed versus traditional resistance-training regimens. Eur J Appl Physiol 112, 3585–3595 (2012). https://doi.org/10.1007/s00421-012-2339-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-012-2339-3