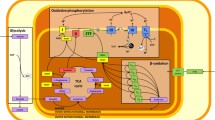
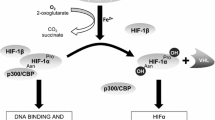
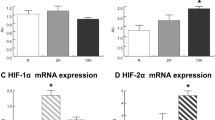
Abstract
An holistic approach for interpreting classical data on the adaptation of the animal and, particularly, of the human body to hypoxic stress was promoted by the discovery of HIF-1, the “master regulator” of cell hypoxic signaling. Mitochondrial production of ROS stabilizes the O2-regulated HIF-1α subunit of the HIF-1 dimer promoting transaction functions in a large number of potential target genes, activating transcription of sequences into RNA and, eventually, protein production. The aim of the present preliminary study is to assess whether adaptive changes in oxygen sensing and metabolic signaling, particularly in the control of energy turnover known to occur in cultured cells exposed to hypoxia, are detectable also in the muscles of animals and man. For the present analysis, data obtained from the proteome of the rat gastrocnemius and of the vastus lateralis muscle of humans together with functional measurements were compared with homologous data from hypoxic cultured cells. In particular, the following variables were assessed: (1) the role of stress response proteins in the maintenance of ROS homeostasis, (2) the activity of the PDK1 gene on the shunting of pyruvate away from the TCA cycle in rodents and in humans, (3) the COX-4/COX-2 ratio in hypoxic rodents, (4) the overall efficiency of oxidative phosphorylation in humans during exercise in hypoxia, (5) some features of muscle mitochondrial autophagy in humans undergoing subchronic and chronic altitude exposure. Despite the limited number of observations and the differences in the experimental approach, some initial interesting results were obtained encouraging to pursue this innovative effort.








Similar content being viewed by others
References
Bastien GJ, Schepens B, Willems PA, Heglund NC (2005) Energetics of load carrying in Nepalese porters. Science 308:1755
Cerretelli P, Marzorati M, Marconi C (2009) Muscle bioenergetics and metabolic control at altitude. High Alt Med Biol 10:165–174
Conti M, Marconi C, Grassi B, Marzorati M, Cerretelli P (1995) Chronic hypoxia (5050 m) does not affect the rate of readjustment of muscle oxidations. Faseb J 9:A648
De Palma S, Ripamonti M, Viganò A, Moriggi M, Capitanio D, Samaja M, Milano G, Cerretelli P, Wait R, Gelfi C (2007) Metabolic modulation induced by chronic hypoxia in rats using a comparative proteomic analysis of skeletal muscle tissue. J Proteome Res 6:1974–1984
Flück M (2009) Plasticity of the muscle proteome to exercise at altitude. High Alt Med Biol 10:183–193
Fukuda R, Zhang H, Kim JW, Shimoda L, Dang CV, Semenza GL (2007) HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell 129:111–122
Ge RL, Chen QH, Wang LH, Gen D, Yang P, Kubo K, Fujimoto K, Matsuzawa Y, Yoshimura K, Takeoka M et al (1994) Higher exercise performance and lower VO2max in Tibetan than Han residents at 4,700 m altitude. J Appl Physiol 77:684–691
Gelfi C, De Palma S, Ripamonti M, Eberini I, Wait R, Bajracharya A, Marconi C, Schneider A, Hoppeler H, Cerretelli P (2004) New aspects of altitude adaptation in Tibetans: a proteomic approach. Faseb J 18:612–614
Gore CJ, Clark SA, Saunders PU (2007) Nonhematological mechanisms of improved sea-level performance after hypoxic exposure. Med Sci Sports Exerc 39:1600–1609
Green HJ, Sutton J, Young P, Cymerman A, Houston CS (1989) Operation everest II: muscle energetics during maximal exhaustive exercise. J Appl Physiol 66:142–150
Howald H, Pette D, Simoneau JA, Uber A, Hoppeler H, Cerretelli P (1990) Effect of chronic hypoxia on muscle enzyme activities. Int J Sports Med 11(Suppl 1):S10–S14
Hurtado A (1964) Animals in high altitudes: resident man. In: Dill DB, Adolph EF, Wilberg CG (eds) Handbook of physiology, sect 4 “Adaptation to the environment.” Am Physiol Soc, Washington, DC, pp 843–860
Hurtado A,Velasquez T, Reynafarje C Lozano R Chavez R et al. (1956)Mechanisms of natural acclimatization. Studies on the native resident of Morococha, Perù, at an altitude of 14,900 feet. Rept. 56–1, School of Aviation Medicine, USAF, Randolph Field, TX
Jewell UR, Kvietikova I, Scheid A, Bauer C, Wenger RH, Gassmann M (2001) Induction of HIF-1 alpha in response to hypoxia is instantaneous. Faseb J 15:1312–1314
Johnson F, Giulivi C (2005) Superoxide dismutases and their impact upon human health. Mol Aspects Med 26:340–352
Kim JW, Tchernyshyov I, Semenza GL, Dang CV (2006) HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab 3:177–185
Lundby C, Calbet JA, Sander M, van Hall G, Mazzeo RS, Stray-Gundersen J, Stager JM, Chapman RF, Saltin B, Levine BD (2007) Exercise economy does not change after acclimatization to moderate to very high altitude. Scand J Med Sci Sports 17:281–291
Marconi C, Marzorati M, Sciuto D, Ferri A, Cerretelli P (2005) Economy of locomotion in high-altitude Tibetan migrants exposed to normoxia. J Physiol 569:667–675
Martinelli M, Winterhalder R, Cerretelli P, Howald H, Hoppeler H (1990) Muscle lipofuscin content and satellite cell volume is increased after high altitude exposure in humans. Experientia 46:672–676
Minetti AE, Formenti F, Ardigò LP (2006) Himalayan porter’s specialization: metabolic power, economy, efficiency and skill. Proc Biol Sci 273:2791–2797
Molé PA, Chung Y, Tran TK, Sailasuta N, Hurd R, Jue T (1999) Myoglobin desaturation with exercise intensity in human gastrocnemius muscle. Am J Physiol 277:R173–R180
Niu W, Wu Y, Li B, Chen N, Song S (1995) Effects of long-term acclimatization in lowlanders migrating to high altitude: comparison with high altitude residents. Eur J Appl Physiol Occup Physiol 71:543–548
Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC (2006) HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab 3:187–197
Powers SK, Jackson MJ (2008) Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev 88:1243–1276
Powers SK, Kavazis AN, McClung M (2007) Oxidative stress and disuse muscle atrophy. J Appl Physiol 102:2389–2397
Raisanen SR, Lehenkari P, Tasanen M, Rahkila P, Harkonen PL, Vaananen HK (1999) Carbonic anhydrase III protects cells from hydrogen peroxide-induced apoptosis. Faseb J 13:513–522
Reynafarje B (1962) Myoglobin content and enzymatic activity of human skeletal muscle–their relation with the process of adaptation to high altitude. Tech Doc Rep SAMTDR USAF Sch Aerosp Med SAM-TDR-62-89, p 8
Richardson RS, Noyszewski EA, Kendrick KF, Leigh JS, Wagner PD (1995) Myoglobin O2 desaturation during exercise: evidence of limited O2 transport. J Clin Invest 96:1916–1926
Ripamonti M, Viganò A, Moriggi M, Milano G, von Segesser LK, Samaja M, Gelfi C (2006) Cytochrome c oxidase expression in chronic and intermittent hypoxia rat gastrocnemius muscle quantitated by CE. Electrophoresis 27:3897–3903
Robach P, Cairo G, Gelfi C, Bernuzzi F, Pilegaard H, Viganò A, Santambrogio P, Cerretelli P, Calbet JA, Moutereau S, Lundby C (2007) Strong iron demand during hypoxia-induced erythropoiesis is associated with down-regulation of iron-related proteins and myoglobin in human skeletal muscle. Blood 109:4724–4731
Semenza GL (2007) Oxygen-dependent regulation of mitochondrial respiration by hypoxia-inducible factor 1. Biochem J 405:1–9
Semenza GL (2009) Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology (Bethesda) 24:97–106
Semenza GL, Wang GL (1992) A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol 12:5447–5454
Sharma R, Yang Y, Sharma A, Awasthi S, Awasthi YC (2004) Antioxidant role of glutathione S-transferases: protection against oxidant toxicity and regulation of stress-mediated apoptosis. Antioxid Redox Signal 6:289–300
Taylor CT (2008) Mitochondria and cellular oxygen sensing in the HIF pathway. Biochem J 409:19–26
Viganò A, Ripamonti M, De Palma S, Capitanio D, Vasso M, Wait R, Lundby C, Cerretelli P, Gelfi C (2008) Proteins modulation in human skeletal muscle in the early phase of adaptation to hypobaric hypoxia. Proteomics 8:4668–4679
Wang GL, Jiang BH, Rue EA, Semenza GL (1995) Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA 92:5510–5514
Yu AY, Frid MG, Shimoda LA, Wiener CM, Stenmark K, Semenza GL (1998) Temporal, spatial, and oxygen-regulated expression of hypoxia-inducible factor-1 in the lung. Am J Physiol 275:L818–L826
Yuan G, Nanduri J, Khan S, Semenza GL, Prabhakar NR (2008) Induction of HIF-1alpha expression by intermittent hypoxia: involvement of NADPH oxidase, Ca2+ signaling, prolyl hydroxylases, and mTOR. J Cell Physiol 217:674–685
Zhang H, Gao P, Fukuda R, Kumar G, Krishnamachary B, Zeller KI, Dang CV, Semenza GL (2007) HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity. Cancer Cell 11:407–420
Zhang H, Bosch-Marce M, Shimoda LA, Tan YS, Baek JH, Wesley JB, Gonzalez FJ, Semenza GL (2008) Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J Biol Chem 283:10892–10903
Acknowledgments
This work was partially supported by the Agenzia Spaziale Italiana (ASI) (to P·C.) and by the Italian Ministry of University and Scientific Research (Grant FIRB RBR NO7BMCT) to C.G.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Susan Ward.
This article is published as part of the Special Issue dedicated to Pietro di Prampero, formerly Editor-in-Chief of EJAP.
Rights and permissions
About this article
Cite this article
Cerretelli, P., Gelfi, C. Energy metabolism in hypoxia: reinterpreting some features of muscle physiology on molecular grounds. Eur J Appl Physiol 111, 421–432 (2011). https://doi.org/10.1007/s00421-010-1399-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-010-1399-5