Abstract
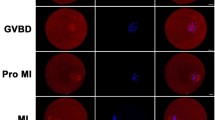
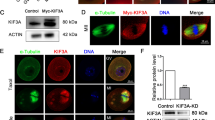
WASP homolog associated with actin, membranes and microtubules (WHAMM) is a newly discovered nucleation-promoting factor that links actin and microtubule cytoskeleton and regulates transport from the endoplasmic reticulum to the Golgi apparatus. However, knowledge of WHAMM is limited to interphase somatic cells. In this study, we examined its localization and function in mouse oocytes during meiosis. Immunostaining showed that in the germinal vesicle (GV) stage, there was no WHAMM signal; after meiosis resumption, WHAMM was associated with the spindle at prometaphase I (Pro MI), metaphase I (MI), telophase I (TI) and metaphase II (MII) stages. Nocodazole and taxol treatments showed that WHAMM was localized around the MI spindle. Depletion of WHAMM by microinjection of specific short interfering (si)RNA into the oocyte cytoplasm resulted in failure of spindle migration, disruption of asymmetric cytokinesis and a decrease in the first polar body extrusion rate during meiotic maturation. Moreover, actin cap formation was also disrupted after WHAMM depletion, confirming the failure of spindle migration. Taken together, our data suggest that WHAMM is required for peripheral spindle migration and asymmetric cytokinesis during mouse oocyte maturation.





Similar content being viewed by others
Abbreviations
- WHAMM:
-
WASP homolog associated with actin, membranes and microtubules
- PB:
-
Polar body
- PB1:
-
First PB
- NPF:
-
Nucleation-promoting factor
- GV:
-
Germinal vesicle
- Pro MI:
-
Prometaphase I
- MI:
-
Metaphase I
- TI:
-
Telophase I
- MII:
-
Metaphase II
- ER:
-
Endoplasmic reticulum
References
Azoury J, Lee KW, Georget V, Rassinier P, Leader B, Verlhac MH (2008) Spindle positioning in mouse oocytes relies on a dynamic meshwork of actin filaments. Curr Biol 18(19):1514–1519. doi:10.1016/j.cub.2008.08.044
Bezanilla M, Wadsworth P (2009) Spindle positioning: actin mediates pushing and pulling. Curr Biol 19(4):R168–R169. doi:10.1016/j.cub.2008.12.026
Brunet S, Maro B (2005) Cytoskeleton and cell cycle control during meiotic maturation of the mouse oocyte: integrating time and space. Reproduction 130(6):801–811. doi:10.1530/rep.1.00364
Brunet S, Verlhac MH (2011) Positioning to get out of meiosis: the asymmetry of division. Hum Reprod Update 17(1):68–75. doi:10.1093/humupd/dmq044
Calarco PG (2005) The role of microfilaments in early meiotic maturation of mouse oocytes. Microsc Microanal Off J Microsc Soc Am Microbeam Anal Soc Microsc Soc Can 11(2):146–153. doi:10.1017/S1431927605050154
Calarco-Gillam PD, Siebert MC, Hubble R, Mitchison T, Kirschner M (1983) Centrosome development in early mouse embryos as defined by an autoantibody against pericentriolar material. Cell 35(3 Pt 2):621–629
Campellone KG, Welch MD (2010) A nucleator arms race: cellular control of actin assembly. Nat Rev Mol Cell Biol 11(4):237–251. doi:10.1038/nrm2867
Campellone KG, Webb NJ, Znameroski EA, Welch MD (2008) WHAMM is an Arp2/3 complex activator that binds microtubules and functions in ER to Golgi transport. Cell 134(1):148–161. doi:10.1016/j.cell.2008.05.032
Carlier MF, Pantaloni D (2007) Control of actin assembly dynamics in cell motility. J Biol Chem 282(32):23005–23009. doi:10.1074/jbc.R700020200
Castano E, Philimonenko VV, Kahle M, Fukalova J, Kalendova A, Yildirim S, Dzijak R, Dingova-Krasna H, Hozak P (2010) Actin complexes in the cell nucleus: new stones in an old field. Histochem Cell Biol 133(6):607–626. doi:10.1007/s00418-010-0701-2
Cowan CR, Hyman AA (2004) Asymmetric cell division in C. elegans: cortical polarity and spindle positioning. Annu Rev Cell Dev Biol 20:427–453. doi:10.1146/annurev.cellbio.19.111301.113823
Deng M, Kishikawa H, Yanagimachi R, Kopf GS, Schultz RM, Williams CJ (2003) Chromatin-mediated cortical granule redistribution is responsible for the formation of the cortical granule-free domain in mouse eggs. Dev Biol 257(1):166–176
Ding L, Pan R, Huang X, Wang JX, Shen YT, Xu L, Zhang Y, Liu Y, He XQ, Yang XJ, Qi ZQ, Wang HL (2012) Changes in histone acetylation during oocyte meiotic maturation in the diabetic mouse. Theriogenology 78(4):784–792. doi:10.1016/j.theriogenology.2012.03.026
Disanza A, Scita G (2008) Cytoskeletal regulation: coordinating actin and microtubule dynamics in membrane trafficking. Curr Biol 18(18):R873–R875. doi:10.1016/j.cub.2008.07.059
Huang X, Tong JS, Wang ZB, Yang CR, Qi ST, Guo L, Ouyang YC, Quan S, Sun QY, Qi ZQ, Huang RX, Wang HL (2011a) JNK2 participates in spindle assembly during mouse oocyte meiotic maturation. Microsc Microanal Off J Microsc Soc Am Microbeam Anal Soc Microsc Soc Can 17(2):197–205. doi:10.1017/S1431927610094456
Huang X, Wang HL, Qi ST, Wang ZB, Tong JS, Zhang QH, Ouyang YC, Hou Y, Schatten H, Qi ZQ, Sun QY (2011b) DYNLT3 is required for chromosome alignment during mouse oocyte meiotic maturation. Reprod Sci 18(10):983–989. doi:10.1177/1933719111401664
Kaksonen M, Toret CP, Drubin DG (2006) Harnessing actin dynamics for clathrin-mediated endocytosis. Nat Rev Mol Cell Biol 7(6):404–414. doi:10.1038/nrm1940
Kollmar M, Lbik D, Enge S (2012) Evolution of the eukaryotic ARP2/3 activators of the WASP family: WASP, WAVE, WASH, and WHAMM, and the proposed new family members WAWH and WAML. BMC Res Notes 5:88. doi:10.1186/1756-0500-5-88
Kutsuna H, Suzuki K, Kamata N, Kato T, Hato F, Mizuno K, Kobayashi H, Ishii M, Kitagawa S (2004) Actin reorganization and morphological changes in human neutrophils stimulated by TNF, GM-CSF, and G-CSF: the role of MAP kinases. Am J Physiol Cell Physiol 286(1):C55–C64. doi:10.1152/ajpcell.00131.2003
Kwon S, Shin H, Lim HJ (2010) Dynamic interaction of formin proteins and cytoskeleton in mouse oocytes during meiotic maturation. Mol Hum Reprod. doi:10.1093/molehr/gaq088
Leader B, Lim H, Carabatsos MJ, Harrington A, Ecsedy J, Pellman D, Maas R, Leder P (2002) Formin-2, polyploidy, hypofertility and positioning of the meiotic spindle in mouse oocytes. Nat Cell Biol 4(12):921–928. doi:10.1038/ncb880
Li H, Guo F, Rubinstein B, Li R (2008) Actin-driven chromosomal motility leads to symmetry breaking in mammalian meiotic oocytes. Nat Cell Biol 10(11):1301–1308. doi:10.1038/ncb1788
Longo FJ, Chen DY (1985) Development of cortical polarity in mouse eggs: involvement of the meiotic apparatus. Dev Biol 107(2):382–394
Matsumura F (2005) Regulation of myosin II during cytokinesis in higher eukaryotes. Trends Cell Biol 15(7):371–377. doi:10.1016/j.tcb.2005.05.004
Pollard TD (2007) Regulation of actin filament assembly by Arp2/3 complex and formins. Annu Rev Biophys Biomol Struct 36:451–477. doi:10.1146/annurev.biophys.35.040405.101936
Rottner K, Hanisch J, Campellone KG (2010) WASH, WHAMM and JMY: regulation of Arp2/3 complex and beyond. Trends Cell Biol 20(11):650–661. doi:10.1016/j.tcb.2010.08.014
Schatten H (2008) The mammalian centrosome and its functional significance. Histochem Cell Biol 129(6):667–686. doi:10.1007/s00418-008-0427-6
Schatten G, Simerly C, Schatten H (1985) Microtubule configurations during fertilization, mitosis, and early development in the mouse and the requirement for egg microtubule-mediated motility during mammalian fertilization. Proc Natl Acad Sci USA 82(12):4152–4156
Schatten H, Schatten G, Mazia D, Balczon R, Simerly C (1986) Behavior of centrosomes during fertilization and cell division in mouse oocytes and in sea urchin eggs. Proc Natl Acad Sci USA 83(1):105–109
Stradal TE, Scita G (2006) Protein complexes regulating Arp2/3-mediated actin assembly. Curr Opin Cell Biol 18(1):4–10. doi:10.1016/j.ceb.2005.12.003
Sun QY, Schatten H (2006) Regulation of dynamic events by microfilaments during oocyte maturation and fertilization. Reproduction 131(2):193–205. doi:10.1530/rep.1.00847
Sun SC, Sun QY, Kim NH (2011a) JMY is required for asymmetric division and cytokinesis in mouse oocytes. Mol Hum Reprod. doi:10.1093/molehr/gar006
Sun SC, Wang ZB, Xu YN, Lee SE, Cui XS, Kim NH (2011b) Arp2/3 complex regulates asymmetric division and cytokinesis in mouse oocytes. PLoS ONE 6(4):e18392. doi:10.1371/journal.pone.0018392
Van Blerkom J, Bell H (1986) Regulation of development in the fully grown mouse oocyte: chromosome-mediated temporal and spatial differentiation of the cytoplasm and plasma membrane. J Embryol Exp Morphol 93:213–238
Verlhac MH, Lefebvre C, Guillaud P, Rassinier P, Maro B (2000) Asymmetric division in mouse oocytes: with or without Mos. Curr Biol 10(20):1303–1306
Yi K, Unruh JR, Deng M, Slaughter BD, Rubinstein B, Li R (2011) Dynamic maintenance of asymmetric meiotic spindle position through Arp2/3-complex-driven cytoplasmic streaming in mouse oocytes. Nat Cell Biol 13(10):1252–1258. doi:10.1038/ncb2320
Zhang CH, Wang ZB, Quan S, Huang X, Tong JS, Ma JY, Guo L, Wei YC, Ouyang YC, Hou Y, Xing FQ, Sun QY (2011) GM130, a cis-Golgi protein, regulates meiotic spindle assembly and asymmetric division in mouse oocyte. Cell Cycle 10(11):1861–1870
Acknowledgments
We thank Ying-Ying LIN, Qing LI, Xiao-Hong HUANG, Yue-Hong MA and Ya-Nan ZHANG for their helpful discussions and technical assistance. This work was supported by grants from the National Natural Science Foundation of China (No. 31201695) and Major State Scientific Research Program of China (No.2012CBA01303).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Huang, X., Ding, L., Pan, R. et al. WHAMM is required for meiotic spindle migration and asymmetric cytokinesis in mouse oocytes. Histochem Cell Biol 139, 525–534 (2013). https://doi.org/10.1007/s00418-012-1051-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-012-1051-z