Abstract
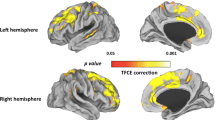
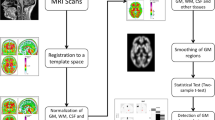
Gray matter (GM) volume deficits have been described in patients with schizophrenia (Sz) and bipolar disorder (BD), but to date, few studies have directly compared GM volumes between these syndromes with methods allowing for whole-brain comparisons. We have used structural magnetic resonance imaging (MRI) and voxel-based morphometry (VBM) to compare GM volumes between 38 Sz and 19 BD chronic patients. We also included 24 healthy controls. The results revealed a widespread cortical (dorsolateral and medial prefrontal and precentral) and cerebellar deficit as well as GM deficits in putamen and thalamus in Sz when compared to BD patients. Besides, a subcortical GM deficit was shown by Sz and BD groups when compared to the healthy controls, although a putaminal reduction was only evident in the Sz patients. In this comparison, the BD patients showed a limited cortical and subcortical GM deficit. These results support a partly different pattern of GM deficits associated to chronic Sz and chronic BD, with some degree of overlapping.



Similar content being viewed by others
References
Adler CM, DelBello MP, Jarvis K, Levine A, Adams J, Strakowski SM (2007) Voxel-based study of structural changes in first-episode patients with bipolar disorder. Biol Psychiatry 61:776–781
Altshuler LL, Bartzokis G, Grieder T, Curran J, Jimenez T, Leight K, Wilkins J, Gerner R, Mintz J (2000) An MRI study of temporal lobe structures in men with bipolar disorder or schizophrenia. Biol Psychiatry 48:147–162
Andreasen NC, Paradiso S, OL DS (1998) “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull 24:203–218
Arnone D, Cavanagh J, Gerber D, Lawrie SM, Ebmeier KP, McIntosh AM (2009) Magnetic resonance imaging studies in bipolar disorder and schizophrenia: meta-analysis. Br J Psychiatry 195:194–201
Ashburner J, Friston KJ (2005) Unified segmentation. Neuroimage 26:839–851
Ashburner J, Friston KJ (2000) Voxel-based morphometry–the methods. Neuroimage 11:805–821
Badcock JC, Michiel PT, Rock D (2005) Spatial working memory and planning ability: contrasts between schizophrenia and bipolar I disorder. Cortex 41:753–763
Ballmaier M, Schlagenhauf F, Toga AW, Gallinat J, Koslowski M, Zoli M, Hojatkashani C, Narr KL, Heinz A (2008) Regional patterns and clinical correlates of basal ganglia morphology in non-medicated schizophrenia. Schizophr Res
Baumann B, Bogerts B (1999) The pathomorphology of schizophrenia and mood disorders: similarities and differences. Schizophr Res 39:141–148 discussion 162
Berrettini W (2003) Evidence for shared susceptibility in bipolar disorder and schizophrenia. Am J Med Genet C Semin Med Genet 123:59–64
Bora E, Fornito A, Yucel M, Pantelis C Voxelwise meta-analysis of gray matter abnormalities in bipolar disorder. Biol Psychiatry
Buchsbaum MS, Shihabuddin L, Brickman AM, Miozzo R, Prikryl R, Shaw R, Davis K (2003) Caudate and putamen volumes in good and poor outcome patients with schizophrenia. Schizophr Res 64:53–62
Byne W, Buchsbaum MS, Mattiace LA, Hazlett EA, Kemether E, Elhakem SL, Purohit DP, Haroutunian V, Jones L (2002) Postmortem assessment of thalamic nuclear volumes in subjects with schizophrenia. Am J Psychiatry 159:59–65
Cahn W, Pol HE, Bongers M, Schnack HG, Mandl RC, Van Haren NE, Durston S, Koning H, Van Der Linden JA, Kahn RS (2002) Brain morphology in antipsychotic-naive schizophrenia: a study of multiple brain structures. Br J Psychiatry Suppl 43:s66–s72
Cardno AG, Rijsdijk FV, Sham PC, Murray RM, McGuffin P (2002) A twin study of genetic relationships between psychotic symptoms. Am J Psychiatry 159:539–545
Corson PW, Nopoulos P, Andreasen NC, Heckel D, Arndt S (1999) Caudate size in first-episode neuroleptic-naive schizophrenic patients measured using an artificial neural network. Biol Psychiatry 46:712–720
Craddock N, O’Donovan MC, Owen MJ (2005) The genetics of schizophrenia and bipolar disorder: dissecting psychosis. J Med Genet 42:193–204
Chakos MH, Lieberman JA, Bilder RM, Borenstein M, Lerner G, Bogerts B, Wu H, Kinon B, Ashtari M (1994) Increase in caudate nuclei volumes of first-episode schizophrenic patients taking antipsychotic drugs. Am J Psychiatry 151:1430–1436
Danos P, Baumann B, Kramer A, Bernstein HG, Stauch R, Krell D, Falkai P, Bogerts B (2003) Volumes of association thalamic nuclei in schizophrenia: a postmortem study. Schizophr Res 60:141–155
Deng MY, McAlonan GM, Cheung C, Chiu CP, Law CW, Cheung V, Sham PC, Chen EY, Chua SE (2009) A naturalistic study of grey matter volume increase after early treatment in anti-psychotic naive, newly diagnosed schizophrenia. Psychopharmacology (Berl) 206:437–446
Dorph-Petersen KA, Pierri JN, Perel JM, Sun Z, Sampson AR, Lewis DA (2005) The influence of chronic exposure to antipsychotic medications on brain size before and after tissue fixation: a comparison of haloperidol and olanzapine in macaque monkeys. Neuropsychopharmacology 30:1649–1661
Farrow TF, Whitford TJ, Williams LM, Gomes L, Harris AW (2005) Diagnosis-related regional gray matter loss over two years in first episode schizophrenia and bipolar disorder. Biol Psychiatry 58:713–723
First MB, Spitzer RL, Gibbon M, Williams JB (1997) Structured Clinical Interview. American Psychiatric Press, Washington
Friston KJ (2003) Introduction: Experimental design and statistical parametric mapping. In: Frackowiak RSJ, Friston KJ, Frith C, Dolan R, Friston KJ, Price CJ, Zeki S, Ashburner J, Penny WD (eds) Human brain function. Academic Press, San Diego
Glenthoj A, Glenthoj BY, Mackeprang T, Pagsberg AK, Hemmingsen RP, Jernigan TL, Baare WF (2007) Basal ganglia volumes in drug-naive first-episode schizophrenia patients before and after short-term treatment with either a typical or an atypical antipsychotic drug. Psychiatry Res 154:199–208
Gur RE, Cowell P, Turetsky BI, Gallacher F, Cannon T, Bilker W, Gur RC (1998) A follow-up magnetic resonance imaging study of schizophrenia. Relationship of neuroanatomical changes to clinical and neurobehavioral measures. Arch Gen Psychiatry 55:145–152
Ha TH, Ha K, Kim JH, Choi JE (2009) Regional brain gray matter abnormalities in patients with bipolar II disorder: a comparison study with bipolar I patients and healthy controls. Neurosci Lett 456:44–48
Hirayasu Y, McCarley RW, Salisbury DF, Tanaka S, Kwon JS, Frumin M, Snyderman D, Yurgelun-Todd D, Kikinis R, Jolesz FA, Shenton ME (2000) Planum temporale and Heschl gyrus volume reduction in schizophrenia: a magnetic resonance imaging study of first-episode patients. Arch Gen Psychiatry 57:692–699
Hirayasu Y, Tanaka S, Shenton ME, Salisbury DF, DeSantis MA, Levitt JJ, Wible C, Yurgelun-Todd D, Kikinis R, Jolesz FA, McCarley RW (2001) Prefrontal gray matter volume reduction in first episode schizophrenia. Cereb Cortex 11:374–381
Hollingshead A, Frederick R (1953) Social stratification and psychiatric disorders. Am Soc Rev 18:163–189
Janssen J, Reig S, Parellada M, Moreno D, Graell M, Fraguas D, Zabala A, Garcia Vazquez V, Desco M, Arango C (2008) Regional gray matter volume deficits in adolescents with first-episode psychosis. J Am Acad Child Adolesc Psychiatry 47:1311–1320
Jayakumar PN, Venkatasubramanian G, Gangadhar BN, Janakiramaiah N, Keshavan MS (2005) Optimized voxel-based morphometry of gray matter volume in first-episode, antipsychotic-naive schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 29:587–591
Kasai K, Shenton ME, Salisbury DF, Onitsuka T, Toner SK, Yurgelun-Todd D, Kikinis R, Jolesz FA, McCarley RW (2003) Differences and similarities in insular and temporal pole MRI gray matter volume abnormalities in first-episode schizophrenia and affective psychosis. Arch Gen Psychiatry 60:1069–1077
Kawasaki Y, Suzuki M, Nohara S, Hagino H, Takahashi T, Matsui M, Yamashita I, Chitnis XA, McGuire PK, Seto H, Kurachi M (2004) Structural brain differences in patients with schizophrenia and schizotypal disorder demonstrated by voxel-based morphometry. Eur Arch Psychiatry Clin Neurosci 254:406–414
Kemether EM, Buchsbaum MS, Byne W, Hazlett EA, Haznedar M, Brickman AM, Platholi J, Bloom R (2003) Magnetic resonance imaging of mediodorsal, pulvinar, and centromedian nuclei of the thalamus in patients with schizophrenia. Arch Gen Psychiatry 60:983–991
Kempton MJ, Geddes JR, Ettinger U, Williams SC, Grasby PM (2008) Meta-analysis, database, and meta-regression of 98 structural imaging studies in bipolar disorder. Arch Gen Psychiatry 65:1017–1032
Keshavan MS, Rosenberg D, Sweeney JA, Pettegrew JW (1998) Decreased caudate volume in neuroleptic-naive psychotic patients. Am J Psychiatry 155:774–778
Ketter TA, Wang PW, Becker OV, Nowakowska C, Yang Y (2004) Psychotic bipolar disorders: dimensionally similar to or categorically different from schizophrenia? J Psychiatr Res 38:47–61
Koo MS, Levitt JJ, McCarley RW, Seidman LJ, Dickey CC, Niznikiewicz MA, Voglmaier MM, Zamani P, Long KR, Kim SS, Shenton ME (2006) Reduction of caudate nucleus volumes in neuroleptic-naive female subjects with schizotypal personality disorder. Biol Psychiatry 60:40–48
Kubicki M, Shenton ME, Salisbury DF, Hirayasu Y, Kasai K, Kikinis R, Jolesz FA, McCarley RW (2002) Voxel-based morphometric analysis of gray matter in first episode schizophrenia. Neuroimage 17:1711–1719
Lee CU, Shenton ME, Salisbury DF, Kasai K, Onitsuka T, Dickey CC, Yurgelun-Todd D, Kikinis R, Jolesz FA, McCarley RW (2002) Fusiform gyrus volume reduction in first-episode schizophrenia: a magnetic resonance imaging study. Arch Gen Psychiatry 59:775–781
Levitt JJ, McCarley RW, Dickey CC, Voglmaier MM, Niznikiewicz MA, Seidman LJ, Hirayasu Y, Ciszewski AA, Kikinis R, Jolesz FA, Shenton ME (2002) MRI study of caudate nucleus volume and its cognitive correlates in neuroleptic-naive patients with schizotypal personality disorder. Am J Psychiatry 159:1190–1197
Lim KO, Rosenbloom MJ, Faustman WO, Sullivan EV, Pfefferbaum A (1999) Cortical gray matter deficit in patients with bipolar disorder. Schizophr Res 40:219–227
Manji HK, Moore GJ, Chen G (2000) Clinical and preclinical evidence for the neurotrophic effects of mood stabilizers: implications for the pathophysiology and treatment of manic-depressive illness. Biol Psychiatry 48:740–754
Marder SR (1999) An approach to treatment resistance in schizophrenia. Br J Psychiatry Suppl 37:19–22
McClure RK, Phillips I, Jazayerli R, Barnett A, Coppola R, Weinberger DR (2006) Regional change in brain morphometry in schizophrenia associated with antipsychotic treatment. Psychiatry Res 148:121–132
McDonald C, Bullmore E, Sham P, Chitnis X, Suckling J, MacCabe J, Walshe M, Murray RM (2005) Regional volume deviations of brain structure in schizophrenia and psychotic bipolar disorder: computational morphometry study. Br J Psychiatry 186:369–377
McDonald C, Bullmore ET, Sham PC, Chitnis X, Wickham H, Bramon E, Murray RM (2004) Association of genetic risks for schizophrenia and bipolar disorder with specific and generic brain structural endophenotypes. Arch Gen Psychiatry 61:974–984
McDonald C, Marshall N, Sham PC, Bullmore ET, Schulze K, Chapple B, Bramon E, Filbey F, Quraishi S, Walshe M, Murray RM (2006) Regional brain morphometry in patients with schizophrenia or bipolar disorder and their unaffected relatives. Am J Psychiatry 163:478–487
McDonald C, Zanelli J, Rabe-Hesketh S, Ellison-Wright I, Sham P, Kalidindi S, Murray RM, Kennedy N (2004) Meta-analysis of magnetic resonance imaging brain morphometry studies in bipolar disorder. Biol Psychiatry 56:411–417
McIntosh AM, Job DE, Moorhead TW, Harrison LK, Forrester K, Lawrie SM, Johnstone EC (2004) Voxel-based morphometry of patients with schizophrenia or bipolar disorder and their unaffected relatives. Biol Psychiatry 56:544–552
McIntosh AM, Job DE, Moorhead WJ, Harrison LK, Whalley HC, Johnstone EC, Lawrie SM (2006) Genetic liability to schizophrenia or bipolar disorder and its relationship to brain structure. Am J Med Genet B Neuropsychiatr Genet 141B:76–83
Mechelli A, Price CJ, Friston KJ, Ashburner J (2005) Voxel-based morphometry of the human brain: methods and applications. Curr Med Imaging Rev 1(2):105–113
Meda SA, Giuliani NR, Calhoun VD, Jagannathan K, Schretlen DJ, Pulver A, Cascella N, Keshavan M, Kates W, Buchanan R, Sharma T, Pearlson GD (2008) A large scale (N = 400) investigation of gray matter differences in schizophrenia using optimized voxel-based morphometry. Schizophr Res 101:95–105
Meisenzahl EM, Koutsouleris N, Bottlender R, Scheuerecker J, Jager M, Teipel SJ, Holzinger S, Frodl T, Preuss U, Schmitt G, Burgermeister B, Reiser M, Born C, Moller HJ (2008) Structural brain alterations at different stages of schizophrenia: a voxel-based morphometric study. Schizophr Res 104:44–60
Molina V, Hernández JA, Sanz J, Paniagua JC, Hernádez AI, Martín C, Matías J, Calama J, Bote B (2010) Subcortical and cortical gray matter differences between kraepelinian and non kraepelinian schizophrenia patients identified using voxel-based morphometry. Psychiatry Res: Neuroimaging 184:16–22
Molina V, Sanchez J, Sanz J, Reig S, Benito C, Leal I, Sarramea F, Rebolledo R, Palomo T, Desco M (2007) Dorsolateral prefrontal N-acetyl-aspartate concentration in male patients with chronic schizophrenia and with chronic bipolar disorder. Eur Psychiatry 22:505–512
Murray RM, Sham P, Van Os J, Zanelli J, Cannon M, McDonald C (2004) A developmental model for similarities and dissimilarities between schizophrenia and bipolar disorder. Schizophr Res 71:405–416
Nugent AC, Milham MP, Bain EE, Mah L, Cannon DM, Marrett S, Zarate CA, Pine DS, Price JL, Drevets WC (2005) Cortical abnormalities in bipolar disorder investigated with MRI and voxel-based morphometry. Neuroimage
Pantelis C, Velakoulis D, McGorry PD, Wood SJ, Suckling J, Phillips LJ, Yung AR, Bullmore ET, Brewer W, Soulsby B, Desmond P, McGuire PK (2003) Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet 361:281–288
Papiol S, Rosa A, Gutierrez B, Martin B, Salgado P, Catalan R, Arias B, Fananas L (2004) Interleukin-1 cluster is associated with genetic risk for schizophrenia and bipolar disorder. J Med Genet 41:219–223
Peuskens J (1999) The evolving definition of treatment resistance. J Clin Psychiatry 60(Suppl 12):4–8
Sassi RB, Nicoletti M, Brambilla P, Mallinger AG, Frank E, Kupfer DJ, Keshavan MS, Soares JC (2002) Increased gray matter volume in lithium-treated bipolar disorder patients. Neurosci Lett 329:243–245
Scherk H, Kemmer C, Usher J, Reith W, Falkai P, Gruber O (2008) No change to grey and white matter volumes in bipolar I disorder patients. Eur Arch Psychiatry Clin Neurosci 258:345–349
Shenton ME, Dickey CC, Frumin M, McCarley RW (2001) A review of MRI findings in schizophrenia. Schizophr Res 49:1–52
Shihabuddin L, Buchsbaum MS, Hazlett EA, Haznedar MM, Harvey PD, Newman A, Schnur DB, Spiegel Cohen J, Wei T, Machac J, Knesaurek K, Vallabhajosula S, Biren MA, Ciaravolo TM, Luu Hsia C (1998) Dorsal striatal size, shape, and metabolic rate in never-medicated and previously medicated schizophrenics performing a verbal learning task. Arch Gen Psychiatry 55:235–243
Shihabuddin L, Buchsbaum MS, Hazlett EA, Silverman J, New A, Brickman AM, Mitropoulou V, Nunn M, Fleischman MB, Tang C, Siever LJ (2001) Striatal size and relative glucose metabolic rate in schizotypal personality disorder and schizophrenia. Arch Gen Psychiatry 58:877–884
Stanfield AC, Moorhead TW, Job DE, McKirdy J, Sussmann JE, Hall J, Giles S, Johnstone EC, Lawrie SM, McIntosh AM (2009) Structural abnormalities of ventrolateral and orbitofrontal cortex in patients with familial bipolar disorder. Bipolar Disord 11:135–144
Tamagaki C, Sedvall GC, Jonsson EG, Okugawa G, Hall H, Pauli S, Agartz I (2005) Altered white matter/gray matter proportions in the striatum of patients with schizophrenia: a volumetric MRI study. Am J Psychiatry 162:2315–2321
Wilke M, Kassubek J, Ziyeh S, Schulze-Bonhage A, Huppertz HJ (2003) Automated detection of gray matter malformations using optimized voxel-based morphometry: a systematic approach. Neuroimage 20:330–343
Wright IC, McGuire PK, Poline JB, Travere JM, Murray RM, Frith CD, Frackowiak RS, Friston KJ (1995) A voxel-based method for the statistical analysis of gray and white matter density applied to schizophrenia. Neuroimage 2:244–252
Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET (2000) Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry 157:16–25
Acknowledgments
Supported in part by a Grant from the Fondo de Investigaciones Sanitarias (FIS; PI080017). The FIS had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Molina, V., Galindo, G., Cortés, B. et al. Different gray matter patterns in chronic schizophrenia and chronic bipolar disorder patients identified using voxel-based morphometry. Eur Arch Psychiatry Clin Neurosci 261, 313–322 (2011). https://doi.org/10.1007/s00406-010-0183-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-010-0183-1