Abstract
Purpose
Knowledge of adolescent and adult phenotypes of women with polycystic ovary syndrome (PCOS) might drive opportune management. The aim of this study was to compare metabolic and obesity biomarkers between adolescent and adult women with PCOS.
Methods
This observational study compared biomarkers of obesity and metabolism derangements between adolescent (n = 62) and adult (n = 248) women with PCOS. Predictors of metabolic syndrome (MS) were investigated using univariate and multivariate binary logistic regression analysis.
Results
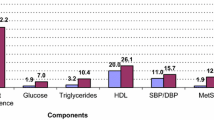
The postmenarcheal age of adolescents was 4.9 ± 0.03 years. Systolic blood pressure was lower in adolescents than in adults (112.3 mmHg vs 117.0 mmHg, p = 0.001) Diastolic blood pressure was also lower in adolescents (70.7 mmHg vs 75.8 mmHg, p < 0.001). Glucose intolerance (12.0% vs 19.3%) and insulin resistance (18.2% vs 17.7%) were similar in both groups (p > 0.05, for comparisons). Impaired fasting glucose was lower in adolescents (1.8% vs 11.6%, p = 0.015). Total cholesterol and low-density lipoprotein cholesterol were lower in adolescents (p < 0.001). MS in adolescents and adults were found in 10.3% and 27.8%, respectively (p = 0.005). Visceral adiposity index (VAI) was a good predictor of MS in both adolescents (OR = 12.2), and adults (OR = 9.7).
Conclusions
Most biomarkers of glucose metabolism abnormalities were similar in adolescents and adults with PCOS. The prevalence of MS was lower in adolescents. VAI was a strong predictor of metabolic syndrome, both in adolescent and adult women with PCOS.
Similar content being viewed by others
Introduction
During women’s reproductive years, the overall prevalence of polycystic ovary syndrome (PCOS) ranges from 5 to 20% [1, 2]. In adolescents, its prevalence has been estimated to range between 0.6% and 12% [3,4,5]. The wide variation is thought to be due to lack of consensus as to diagnostic criteria in adolescents. In population-based studies, PCOS was found in less than 4% of adolescents, aged 15–19 years [3, 4]. Because the diagnostic criteria used in adult women may be confounded by normal pubertal events, the Amsterdam criteria are recommended after adaptation for adolescents, using all three Rotterdam criteria to make a definitive diagnosis of PCOS [6].
In the clinical setting, adolescents with PCOS present with frequent or infrequent menses after two years of menarche or amenorrhea after 15 years of age, persistent acne or hirsutism, overweight or obesity, and signs of insulin resistance, such as acanthosis nigricans [7]. Regarding biochemical hyperandrogenism, total testosterone (T) levels between 42 ng/dL (1.45 nmol/L) and 55 ng/dL (1.90 nmol/L) are considered elevated in this population [6, 8, 9]. Polycystic ovary morphology (PCOM), and size, may be not a helpful markers, as they are in adults [10,11,12,13]. Of note, PCOM is not obligatory among the National Institutes of Health (NIH) diagnostic criteria for PCOS.
Even that a number of studies have reported PCOS in adolescents, studies comparing anthropometric parameters, carbohydrate or lipid biomarkers between adolescents and adults with PCOS are scarce. According to the available data, as regards adolescents, these parameters are either similar [13] or less pronounced [14] to those of adult women. Despite possible changes in PCOS characteristics over time [15], in the present study we compared the clinical and biochemical features between adolescent and adult women with PCOS. Notwithstanding, the limitations of a precise diagnosis of PCOS in adolescents, a comparison of PCOS characteristics between adolescent and adult patients might help to understand the importance of an early diagnosis to tailor optimal and opportune management, and care for adolescents.
Material and methods
Study design and selection criteria
This cross-sectional study enrolled 62 not virgin adolescents diagnosed with PCOS using a combination of all three Rotterdam criteria and 248 women over 19 years of age diagnosed with PCOS using Rotterdam criteria for adults. All patients presented to the Júlio Muller University Hospital or Tropical Institute of Reproductive Medicine, Cuiabá, MT, Brazil, from January 2003 to December 2018. The study was approved by the Committee for Ethics in Research of the Faculty of Medical Sciences, Cuiabá, MT, Brazil (decision No093/FCM/03). Regardless of a prospective approach, some data were not recorded in the patient file. Adolescent women with PCOS aged 15–19 years that had menarche more than 2 years before inclusion in the study. The sample of adults with PCOS was drawn from a population of 836 women with PCOS and generated by random number on the SPSS software.
Patients who had used sex steroids, insulin-sensitizing drugs, or dipeptidyl dipeptidase inhibitors-4 over the previous six months, as well as women who did not fulfil the Rotterdam criteria according to age were excluded. Patients with thyroid stimulating hormone (TSH) levels ≥ 4.2 µIU/L, prolactin (PRL) ≥ 1,086 pmol/L, and 17-hydroxyprogesterone (17-OHP4) ≥ 6 nmol/L were also excluded. The exclusion of classic 3β-hydroxysteroid dehydrogenase (3β-HSD) was based on the threshold serum level of < 11 nmol/L for 17-hydroxypregnenolone (17-OHPE) in normal cycling women [15].
Definitions
Menstrual bleeding was defined according to the FIGO recommendations [16]. In adolescents, frequent menses was defined as cycles shorter than 19 days; infrequent menses was defined as cycles longer than 45 days at least 2 years after menarche, and was defined as amenorrhea as cycles longer than 90 days at any stage after menarche and primary amenorrhea by age 15 or over 3 years post-thelarche [17, 18]. In adults, frequent menses was defined as cycles shorter than 21 days; infrequent menses was defined as cycles longer than 45 days or eight menses per year; and amenorrhea was defined as cycles longer than 90 days at any age. In both groups, obesity was defined using body mass index (BMI) corrected by age using an online program [19, 20].
Because inconsistency between examiners and ethnicity variation, clinical hyperandrogenism was considered present when the patient claimed to have acne or dark thick hair on upper lip or face, chest, abdomen, back or thighs confirmed on physical examination [15, 21]. In adolescents, biochemical hyperandrogenism was defined as the persistence of androgen serum levels exceeding three standard deviation from the mean of normal cycling adolescents as follows: total testosterone ≥ 1.56 nmol/L, free testosterone ≥ 0.8 pmol/dL, and DHEAS ≥ 5.3 µmol/L. In adults, biochemical hyperandrogenemia was defined by the presence of one or more of the following parameters: testosterone (T) ≥ 2.1 nmol/L, free T ≥ 0.027 pmol/L, dehydroepiandrosterone sulfate (DHEAS) ≥ 6.7 µmol/L, androstenedione (A4) ≥ 8.6 nmol/L, and free androgen index (FAI) ≥ 6 [1, 22].
Impaired glucose tolerance (IGT) was defined as fasting plasma glucose between 100 mg/dL (5.5 mmol/L) and 126 mg/dL (6.99 mmol/L); glucose intolerance was defined as 2-h OGTT glucose values between 140 mg/dL (7.8 mmol/L) and 199 mg/dL (11.0 mmol/L) [23]. Type II diabetes mellitus (T2DM) was defined as fasting glucose ≥ 6.99 mmol/L or over 11.1 mmol/L at 120 min after 75 g dextrose ingestion. Insulin resistance was defined using fasting insulin levels > 12.2 µU/mL (84.7 pmol/L); and/or homeostasis model assessment of insulin resistance (HOMA-IR) ≥ 2.7 [24].
In adolescents, elevated blood pressure was defined as systolic (SBP) and diastolic blood pressures (DBP) ≥ 90th percentile of normal cycling adolescents as defined by the Clinical Practice Guideline, American Academy of Pediatrics [25]. Metabolic syndrome (MS) was present when the adolescent met three or more of the following criteria: waist circumference (WC) of at least 90th percentile for age and gender, systolic or diastolic blood pressures at least the 90th percentile for age and gender, fasting glucose of at least 100 mg/dL (5.55 mmol/L), HDL-C ≤ 40 mg/dL (1.03 mmol/L), and fasting triglyceride ≥ 110 mg/dL (1.24 mmol/L) [26]. In adults, MS was diagnosed using the National Cholesterol Education Program (NCEP), Adult Treatment Panel III [27].
Measurement of anthropometric characteristics
All subjects were weighed on an electronic scale. Waist circumference (WC) was measured at the midway point between the lower rib margin and the trocanter crest, and the hip was measured at the widest circumference at the iliac crest. Body mass index (BMI) was calculated as body weight (kg)/height (m2). Lean body mass (LBM) was calculated using the James equation: [1.07 × weight (kg)] −148 × weight (kg)2)/[100 × height (m)2)] [28]. Fat mass (FM) was calculated as body weight minus LBM (BW − LBM). The visceral adiposity index (VAI) was estimated using the equation: WC/[36.58 + (1.89 × BMI)] × (TG / 0.81) × (1.52/HDL-C) [29]. Lipid accumulation product (LAP) was calculated as (WC (cm) − 58) × (TG (mmol) as established for women [30].
Biochemical and hormone analysis
Adolescent and adult PCOS patients with infrequent menses or amenorrhea had their blood collected at any time; however, the results were validated when progesterone levels were less than 6.4 nmol/L. Adults with PCOS who presented with regular cycles were tested in the early follicular phase of the menstrual cycle (days 3–5). Glucose was analyzed using the glucose oxidase technique (Beckman Glucose Analyses, Fullerton, CA, USA). Triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), and total cholesterol (TC) levels were measured using an enzymatic assay (Wiener Laboratorios, Rosário, Argentina). Low-density lipoprotein cholesterol (LDL-C) was calculated as TC − (HDL − C + TG/5) [31].
The oral glucose tolerance test (OGTT) was performed by measuring glucose before and at 30, 60, 90, 120, and 180 min after administration of 75 g oral dextrose. The homeostatic model for insulin resistance (HOMA-IR), tissue sensitivity, to insulin (HOMA-S), and β-cell function (HOMA%B) were calculated using a free online program [32]. All hormone measurements and their error ranges were detailed in a recent publication [15]. Levels of 17-OHPE were measured using an HPLC/MS/MS (Labco Nous Advanced Special Diagnostics, SP, Brazil). Free androgen index (FAI) was calculated as the T (nmol/L)/sex hormone binding globulin (SHBG) (nmol/L) × 100 [26].
Ultrasound
Despite pelvic ultrasound had not been recommended for the diagnosis of PCOS before 8 years post-menarche [12, 13, 33], polycystic ovary morphology (PCOM) was defined by established criteria for adolescents and adults [7, 33, 34] using vaginal transducer with a frequency of 5–8 MHz (Toshiba Xario SSA-660A, Toshiba Medical do Brazil LTDA, Taboão da Serra, SP, Brazil or Voluson® E8, GE Healthcare, United Kingdom). By definition all enrolled adolescents presented PCOM and, among adults, the PCOM was identified in 75.8% of patients.
Statistical analysis
Results were presented as mean \(\stackrel{-}{x}\) and standard deviation (SD) or number and percentage (n%). Comparison of ethnicities was performed using the chi-square test. Presence of galactorrhea between groups was compared using the Fisher exact test. Proportions of impaired glucose tolerance (IGT), fasting insulin, HOMA-IR, HOMA%B, and androgen concentrations between groups were also compared using the chi-square test followed by the Dunn test with Bonferroni adjustments. Because of the unequal sample size baseline differences between two independent continuous variables were adjusted using the Welch test. Powers for all comparisons were verified. Univariate (enter model) and multivariate (forward stepwise model) binary logistic regression analyses were performed to identify potential predictors of MS. The goodness of fit of the models were examined using the Hosmer–Lemeshow test and explained variation was calculated using the Cox-Snell \({R}^{2}\) and Nagelkerke \({R}^{2}\) square tests. All statistical procedures were performed using SPSS version 17 (SPSS, Inc, Chicago, IL, USA). All tests were two-sided, and p-values < 0.05 were considered statistically significant.
Results
Adolescent and adult PCOS patients were on average 16.8 ± 1.6 and 28.2 ± 4.8 years old, respectively (P < 0.001). The age at menarche of adolescents was 11.9 ± 1.3 years; therefore, the mean post-menarcheal age of this group was 4.9 ± 0.03 years. Among adolescents, 65.0% were Caucasians, 11.3% African descendants, and 24.2% were of mixed or other races. Among adults with PCOS, 72.1% were Caucasians, 12.9% were African descendants, and 15.0% were of mixed or other races. Multiethnic women were significantly more prevalent among adolescents (P = 0.015). Systolic blood pressure was lower in adolescents PCOS subjects (112.3 ± 10.9 mmHg) than in adults (117.4 ± 11.6 mmHg, P = 0.011, power = 0.292). Diastolic blood pressure was also lower in adolescents with PCOS (70.7 ± 8.6 mmHg vs 75.8 ± 8.9 mmHg, P < 0.001, power = 0.048).
The comparisons of other clinical characteristics between adolescent and adult PCOS patients are shown in Table 1. Obesity was found in 94 (37.9%) adults and in 12 (21%) adolescents (P = 0.016). Adolescent with PCOS had significantly more acne lesions than did adults with PCOS (P = 0.033). Frequent menses were more prevalent in adolescents (P = 0.015). Attending diagnosis requirement regular cycles were not present in adolescents but were found in 13.7% adults (P = 0.001). The ovaries were slightly smaller in adolescents with PCOS than in adults PCOS patients (9.5 ± 2.1 cm3 vs 10.7 ± 5.1 cm3, P = 0.004) and the uterine size was also lower in adolescents than in adults (42.0 ± 1.3 vs 55.3 ± 2.4 cm3, P < 0.001). The endometria thicknesses were not different between groups, 6.2 ± 2.9 mm in adolescents and 7.0 ± 3.5 mm in adults PCOS patients (P = 0.100).
Impaired fasting glucose (IFG) was found in 01/56 (1.8%) adolescents and in 35/248 (14.1%) adults (P = 0.005). Glucose intolerance was present in 3/25 adolescents (12.0%) and in 48/248 (19.3%) adults (P = 0.589). HOMA-IR ≥ 2.7 was found in 10/55 (18.2%) adolescents and in 44/248 (17.7%) adults (P = 0.938). Using baseline levels of fasting insulin ≥ 12.2 µUI/mL as the cut-off, insulin resistance was present in 20/56 (35.7%) adolescents and in 120/248 (48.4%) adults (P = 0.085). Abnormal beta cell function was similar in adolescent and adult PCOS groups [20/55 (36.4%) vs 70/197 (35.5%); P = 0.912]. Diabetes mellitus was not found in adolescents, but was diagnosed in 11/248 (4.4%) adult women with PCOS (P = 0.129).
Baseline hormone characteristics of adolescent and adults PCOS subjects are compared in Table 2. Concentrations and proportions of those hormones that have the adrenal gland as the principal source tended to be higher in adolescents, but significant statistical differences were demonstrated only for DHEAS and cortisol. The prevalence of higher levels of DHEAS was greater in adolescents (37/56, 66.0%) compared with adults (45/210, 21.4%) with PCOS (P < 0.001). Additionally, the proportion of women with increased T levels was higher in adolescents with PCOS (41/57, 71.9%) compared with adults (91/222, 41.1%) (P < 0.001). FT had equal prevalence in adolescents and adults (34/54, 62.9% and 113/214, 52.8%, respectively, P = 0.180).
Anthropometric measures and metabolic biomarkers between adolescent and adult PCOS women are compared in Table 3. Most of these parameters were significantly lower in adolescent subjects; however, WHR (0.78 ± 0.06 vs 0.80 ± 0.08, P = 0.097), and LBM (kg) (44.2 ± 6.6 vs 46.0 ± 6.2, P = 0.054) were similar in adolescents and adults. Comparisons of WHR, LBM (kg), CI, and VAI between groups presented powers lower than 20%. TC and VLDL-C levels were lower among adolescents with PCOS (P < 0.001), but TG was similar in both PCOS groups. Only the comparisons of fasting glucose and TG were robust with power lower than 20%. Metabolic syndrome was found in 6/58 (10.3%) adolescents and in 69/248 (27.8%) adults (P = 0.005).
Univariate binary logistic regression analyses was performed with MS as the criterion variable. The independent variables that significantly correlated with MS, both in adolescents and adults, are shown in Supplementary Table 1. In adolescents, the multivariate logistic regression analyses using a forward stepwise final model presented a good fit of the data (\({x}^{2}\) = 2.281, P = 0.943). With the inclusion of eight predictor variables (Supplementary Table 1) the regression explained between 46.0% (Cox and Snell R2) and 74.6% (Nagelkerke R2) of the variance of MS. VAI made a statistically significant contribution to the model, indicating that this biomarker is 12.2 times more likely to identify PCOS adolescents with higher risk for the development of MS (Table 4). Figure 1 shows the predictive ability of VAI to predict MS (AUC = 0.941, p = 0.001).
In adults with PCOS, multivariate logistic regression using a forward stepwise model with the inclusion of sixteen predictor variables that have previously show to be good predictors of MS using univariate logistic regression (Supplementary Table 1). The model presented a good fit of the data (\({x}^{2}\) = 3.634, P = 0.889) and explained between 46.4% (Cox and Snell R2) and 67.2% (Nagelkerke R2) of the MS variation. FM, FAI, VAI, and TG were retained in the final model, showing different abilities to predict the development of MS in adults with PCOS (Table 4). Figure 2 shows the abilities of FM(%), FAI, TG, and VAI to predict MS (AUC = 0.901, p < 0.001). VAI was 9.7 fold more likely favor the appearance of MS in the adult PCOS women.
Discussion
The current results comparing clinical, anthropometric and metabolic biomarkers between adolescent and adult women with PCOS are yet conflictants [13, 18]. In the present study, this comparison confirmed to exist significant differences. SBP and DBP were lower in adolescents than in adults. In agreement with the restricted criteria required to make PCOS diagnoses, the prevalence of abnormal menses was higher in adolescents than in adults. The ovarian size was smaller in adolescents than in adults. The presence of acne was higher in the adolescent group, but the presence of hirsutism was similar in both groups.
WHR, and LBM (kg) were similar in adolescents and adults with PCOS. LBM (%) was higher among adolescents, despite the different cut-offs for adolescents and adults used in the present study. The other anthropometric measures were constantly lower in the adolescent group despite low power seen in some comparisons. However, this observation supports a previous study demonstrating that anthropometric parameters have a tendency to worsening with the advancing age in PCOS women [15]. The higher levels of DHEA, DHEAS, and cortisol, for which the adrenal gland is the main source, in adolescents is a novel finding and suggests that adrenarche may be prolonged in adolescents with PCOS. Similarly, higher prevalence of elevated T and A levels were seen in adolescents, despite the different cut-offs used for adolescent and adults. Among metabolism biomarkers, fasting glucose TC, LDL-C were lower in adolescents. After multivariate logistic regression analysis, VAI was shown to be the only strong predictor of the MS in adolescents. In adults, FM (%) and FAI were maintained in the final model as weak predictors of MS but VAI had a strong predictive value for MS in this group of PCOS patients. This observation appears to be novelty.
A few limitations must be considered in the interpretation of the current results. No direct measurement of LDL-C was used despite the inacuracity of the Friedwald equation. Additionally, ultrasound procedures were not reviewed by an independent radiologist. Of note, low power was seen in the anthropometric-metabolic indexes comparisons, probably because the complex equations used for their calculation. Concerns related to androgen measurements must be considered; even previous comparisons of results between the assays used along the sample collection period and liquid chromatography-tandem mass spectrometry have shown good agreement this is a justified limitation of this study [23, 35, 36]. Due to the different sizes of the samples, despite significant statistics differences, low power was seen in some comparisons. Additional examination for the presence of clinical atherosclerosis and coronary diseases was not performed. As strengths, the present study enrolled a large number of well-characterized women in both PCOS groups and clear definitions were provided for primary and secondary variables. Additionally, the differences in sample sizes were adjusted using adequate statistics test. The results have clinical implications and should be applied early for the prevention or therapeutic intervention of PCOS women to prevent future deterioration.
Because menstrual irregularities may be present between 25% and 40% in the first 1–4 years after menarche [37], it is difficult to characterize a specific abnormal menstrual parameter to be used as a criterion to diagnose PCOS in adolescents. In the current study, abnormal menses were assumed to be a criterion to diagnose PCOS when it persisted after 2 years post-menarche, and was associated with hyperandrogenism or increased ovarian volume, as recommended by the Endocrine Society and other researchers [6, 38]. In spite of this drawback, it is important to consider that PCOS has been previously diagnosed in 95% of adolescent with persistent irregular menses [39]. Note that in the current study infrequent menses were nearly four times more prevalent in adolescents than in adults with PCOS, perhaps as a consequence of the differences in the diagnostic criteria.
The higher prevalence of acne in adolescents with PCOS was consistent with results of a recent report [13]. Though acne occurs in a large number of adolescents as a transitory condition, it is of little value in the diagnosis of PCOS [6]. Despite this knowledge, it is worthwhile testing for biochemical hyperandrogenism in cases of persistent acne [40]. Previously, acne was reported to affect 15%–25% of PCOS patients [39, 41], about 20% in adolescents and 15% after their 20 s [42]. However, in the current study acne was more prevalent, 45% of adolescents and 40% of adults with PCOS. Hirsutism was reported to affect up to 75% of women with PCOS [41], but in the current study, hirsutism was found in 45% of adolescents and 55% of adults. Recently, similar results have been reported in adolescents and adults, even though in the present study hirsutism had been taken as a binomial variable and not by the Ferriman–Gallwey score. Of note, there is no separate cut-off for adolescents when the Ferriman–Gallwey score is used [18].
Hyperandrogenism is mandatory to make diagnosis of PCOS in adolescents. Despite assay controversies, biochemical hyperandrogenism is found in 25%–83% of adolescents and in about 70–80% of adults with PCOS [15, 43, 44]. In the current study, based on T, A, and DHEAS levels, biochemical hyperandrogenism was present in more than 70% of adolescents. It is worth noting that the diagnosis of biochemical hyperandrogenism in PCOS adolescents requires well-defined ranges [38, 40, 45, 46]; nevertheless, a cut-off of three standard deviations from the mean of non-PCOS adolescents used in the current study appears to be adequate.
Between 30 and 31% of normal adolescents may have polycystic-appearing ovaries [12, 47]. Therefore, the frequency of multifollicular morphology of ovaries in adolescents limits the use of this criterion to confirm the diagnosis of PCOS in this population [12, 48]. In adolescents, follicular count is not recommended as it is in adults [7], and the ovarian volume should be considered [12, 47, 48]. Others recommend the use of NIH criteria, exempting the obligation of ovarian morphology for PCOS diagnosis in adolescents [39]. Because ovarian volume begins to increase with the onset of puberty and at the age of 16 remains stable, or even decreases thereafter [7], it is expected that the ovaries of adolescents have small volumes, than those of adult ovaries. The findings of the current study are consistent with this rationale.
Approximately 27%–50% of adolescents and 50%–70% of adults were reported to be obeses [14, 49,50,51]. The results of the current study support these findings showing obesity in 21% of adolescents and in 38% of adults. The higher proportion of acanthosis nigricans seen in adolescents was not significant, notwithstanding baseline levels of insulin to be higher in adults. We speculate that other unknown factors may be involved in the appearance of acanthosis. It has been shown that obesity exacerbates the appearance of impaired fasting glucose, glucose intolerance, insulin resistance, arterial hypertension, and metabolic syndrome [14, 23, 49, 52]. Of note, these abnormalities appear early in the second decade of the lives of women with PCOS.
In the current study, abnormalities in glucose metabolism were found in 36% of adolescents and in 48% of adult with PCOS. The risks of glucose intolerance, IR, and T2DM have been reported to be greater in adolescents with PCOS [49, 51, 52]. In obese adolescents with PCOS, glucose metabolism derangements were reported in between 4.5% and 52% [44, 52], and in adults with PCOS between 30 and 40% [53, 54]. Others have reported that adolescents and adults with PCOS may have similar prevalence of IR [52, 55]. In adolescents, IR was reported to be higher in the presence of increased levels of androgen [43, 56]. Contrary to other reports, in the present study, glucose intolerance and IR were higher in adult with PCOS but without statistical significance. Only IFG was significantly higher in adults than in adolescents with PCOS. Our group has demonstrated that TC, LDL-C, IFG, IR, and TG tended to increase over time in women with PCOS [15]. Thus, despite lower levels of fasting glucose, TC, and LDL-C in adolescents in the current study, precocious introduction of physical exercise and changes in the lifestyle to ovoid deterioration in the carbohydrate and lipid metabolism in adolescent women with PCOS are recommended. It must be considered that most markers of carbohydrate metabolism increase in women with PCOS over time [15].
The prevalence of at least one abnormal lipid in adults with PCOS is approximately 70%–75% [57, 58]. In both, adolescents and adults women with PCOS, the major risk for dyslipidemia is the hyperandrogenemic state [14, 22, 46, 56,57,58,59]. In the current study, TC and LDL-C levels were higher in adults; however, HDL-C and TG levels were similar in adolescents and adults with PCOS. Dyslipidemia was reported to favor the appearance of MS in adolescents [14]. In the present study, VAI, combining WC, BMI, HDL-C and TG, was the best predictor of MS in adolescents. By consistency, VAI also was the better predictor of MS in adults with PCOS. The finding that VAI was the best predictor of MS in PCOS seems to be novelty and will be examined in future studies.
In PCOS, MS has been reported in the range of 8%–37% of adolescents [14, 46, 59] and in the range 14%–46% of adults [43, 60,61,62]. In the present study, MS was nearly 2.7 times less prevalent in adolescents than in adults with PCOS. In other Brazilian region, the prevalence of MS in adolescent and adult PCOS patients was 24% and 43%, respectively [60]. After adjusting for BMI, MS was reported to be 4.5 times more likely in adolescents with PCOS than in normal women [14]. This comparison was not done in the present study. The different prevalences of MS in adolescents and adults suggest a worsening over time. In the general population, MS was reported between 12% and 44% [61,62,63].
Conclusions
In conclusion, adolescent and adult patients with PCOS present significant differences in their features. Systolic and diastolic pressures are lower in the adolescents. The higher prevalence of infrequent menses in adolescents indicates differences in the criteria for the diagnosis of PCOS in this group or improvement in the ovarian function over time. The hormones for which the adrenal gland is the main source tended to be higher indicating an extension of adrenarche in adolescents with PCOS. Most biomarkers of glucose metabolism abnormalities are similar in adolescents and adults with PCOS, but prevalences of glucose intolerance and insulin resistance were lower in adolescents. VAI is a good predictor of metabolic syndrome, both in adolescent and adult women with PCOS.
References
Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO (2004) The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab 89:2745–2749. https://doi.org/10.1210/jc.2003-032046
March WA, Moore VM, Wilson KJ, Phillips DIW, Norman RJ, Davies MJ (2010) The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod 25:544–551. https://doi.org/10.1093/humrep/dep399
Hashemipour M, Faghihimani S, Zolfaghary B, Hovsepian S, Ahmadi F, Haghighi S (2004) Prevalence of polycystic ovary syndrome in girls aged 14–18 years in Isfahan. Iran Horm Res 62:278–282. https://doi.org/10.1159/000081842
Christensen SB, Black MH, Smith N, Martinez MM, Jacobsen SJ, Porter AH, Koebnick C (2013) Prevalence of polycystic ovary syndrome in adolescents. Fertil Steril 100:470–477. https://doi.org/10.1016/j.fertnstert.2013.04.001
Singh A, Vijaya K, Laxmi KS (2018) Prevalence of polycystic ovary syndrome among adolescent girls: a prospective study. Int J Reprod Contracept Obstet Gynecol 7:4375–4378
Carmina E, Oberfield SE, Lobo RA (2010) The diagnosis of polycystic ovary syndrome in adolescents. Am J Obstet Gynecol 203:201.e1-201.e2015. https://doi.org/10.1016/j.ajog.2010.03.008
Witchel SF, Oberfield S, Rosenfield RL, Codner E, Bonny A, Ibáñez L, Pena A, Horikawa R, Gomez-Lobo V, Joel D, Tfayli H, Arslanian S, Dabadghao P, Garcia Rudaz C, Lee PA. The diagnosis of polycystic ovary syndrome during adolescence (2015). Horm Res Paediatr. https://doi.org/10.1159/000375530
Brewer M, Pawelczak M, Kessler M, Shah B (2010) A review of polycystic ovarian syndrome in adolescents. Minerva Pediatr 62:459–473
Gambineri A, Fanelli F, Prontera O, Repaci A, Di Dalmazi G, Zanotti L, Pagotto U, Flacco ME, Guidi J, Fava GA, Manzoli L, Pasquali R (2013) Prevalence of hyperandrogenic states in late adolescent and young women: epidemiological survey on italian high-school students. J Clin Endocrinol Metab 98:1641–1650. https://doi.org/10.1210/jc.2012-3537
Venturoli S, Porcu E, Fabbri R, Pluchinotta V, Ruggeri S, Macrelli S, Paradisi R, Flamigni C (1995) Longitudinal change of sonographic ovarian aspects and endocrine parameters in irregular cycles of adolescence. Pediatr Res 38:974–980. https://doi.org/10.1203/00006450-199512000-00024
Rosenfield RL, Ghai K, Ehrmann DA, Barnes RB (2000) Diagnosis of the polycystic ovary syndrome in adolescence: comparison of adolescent and adult hyperandrogenism. J Pediatr Endocrinol Metab 13:1285–1289
Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, Piltonen T, Norman RJ, International PCOS Network (2018) Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril 110:364–379. https://doi.org/10.1016/j.fertnstert.2018.05.004
Zore T, Lizneva D, Brakta S, Walker W, Suturina L, Azziz R (2019) Minimal difference in phenotype between adolescents and young adults with polycystic ovary syndrome. Fertil Steril 111:389–396. https://doi.org/10.1016/j.fertnstert.2018.10.020
Coviello AD, Legro RS, Dunaif A (2006) Adolescent girls with polycystic ovary syndrome have an increased risk of the metabolic syndrome associated with increasing androgen levels independent of obesity and insulin resistance. J Clin Endocrinol Metab 91:492–497. https://doi.org/10.1210/jc.2005-1666
de Medeiros SF, Yamamoto MMW, Souto de Medeiros MA, Barbosa BB, Soares JM, Baracat EC (2020) Changes in clinical and biochemical characteristics of polycystic ovary syndrome with advancing age. Endocr connect 9:74–89. https://doi.org/10.1530/EC-19-0496
Fraser IS, Critchley HO, Broder M, Munro MG (2011) The FIGO recommendations on terminologies and definitions for normal and abnormal uterine bleeding. Sem Reprod Med 29:383–390. https://doi.org/10.1055/s-0031-1287662
Sultan C, Paris F (2006) Clinical expression of polycystic ovary syndrome in adolescent girls. Fertil Steril 86:S6. https://doi.org/10.1016/j.fertnstert.2006.04.015
Peña AS, Witchel SF, Hoeger KM, Oberfield SE, Vogiatzi MG, Misso M, Garad R, Dabadghao P, Teede H (2020) Adolescent polycystic ovary syndrome according to the international evidence-based guideline. BMC Med 18:72. https://doi.org/10.1186/s12916-020-01516-x
WHO Consultation on Obesity (1997): Geneva, Switzerland), World Health Organization. Division of Noncommunicable Diseases & World Health Organization. Programme of Nutrition, Family and Reproductive Health (1998). Obesity: preventing and managing the global epidemic: report of a WHO Consultation on Obesity, Geneva, 3–5 June 1997. World Health Organization. https://apps.who.int/iris/handle/10665/63854. Accessed 02 July 2020
SBMIC 2.7 (2014 – 2020). Calculate your BMI correctly rated according to age and sex. https://www.smartbmicalculator.com. Accessed 02 July 2020
Kristensen SL, Ramlau-Hansen CH, Ernst E, Olsen SF, Bonde JP, Vested A, Toft G (2010) A very large proportion of young Danish women have polycystic ovaries: is a revision of the Rotterdam criteria needed? Hum Reprod 25:3117–3122. https://doi.org/10.1093/humrep/deq273
de Medeiros SF, Barbosa JS, Yamamoto MMW (2015) Comparison of steroidogenic pathways among normoandrogenic and hyperandrogenic polycystic ovary syndrome patients and normal cycling women. J Obstet Gynaecol Res 2015(41):254–263. https://doi.org/10.1111/jog.12524
Carmina E, Bucchieri S, Esposito A, Del Puente A, Mansueto P, Orio F, Di Fede G, Rini G (2007) Abdominal fat quantity and distribution in women with polycystic ovary syndrome and extent of its relation to insulin resistance. J Clin Endocrinol Metab 92:2500–2505. https://doi.org/10.1210/jc.2006-2725
Geloneze B, Vasques AC, Stabe CF, Pareja JC, Rosado LE, Queiroz EC, Tambascia MA, Investigators BRAMS (2009) HOMA1-IR and HOMA2-IR indexes in identifying insulin resistance and metabolic syndrome: Brazilian metabolic syndrome study (BRAMS). Arq Bras Endocrinol Metab 53:281–287. https://doi.org/10.1590/s0004-27302009000200020
Flynn JT, Kaelber DC, Baker-Smith CM, Blowey D, Carroll AE, Daniels SR et al (2017) Clinical practice guideline for screening and management of high blood pressure in children and adolescents. J Pediatr 140:e20171904. https://doi.org/10.1542/peds.2017-1904
Cook S, Auinger P, Li C, Ford ES (2008) Metabolic syndrome rates in United States adolescents, from the national health and nutrition examination survey, 1999–2002. J Pediatr 152:165–170. https://doi.org/10.1016/j.jpeds.2007.06.004
National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) (2002) Third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation 106:3143–3421
James WPT (1976). Research on obesity. A repot of the DHSS/MRC group. Her Majesty’s Stationary. Department of Health and Social Security and Medical Research Council Group. In: (ed). Hher Majesty’s Stationary Office. London: Office. 94. https://doi.org/10.1111/j.1467-3010.1977.tb00966.x
Amato MC, Giordano C (2014) Visceral adiposity index: an indicator of adipose tissue dysfunction. Int J Endocrinol 3:730827. https://doi.org/10.1155/2014/730827
Kahn HS, Valdez R (2003) Metabolic risks identified by the combination of enlarged waist and elevated triacylglycerol concentration. Am J Clin Nutr 78:928–934. https://doi.org/10.1093/ajcn/78.5.928
Friedewald WT, Levy RI, Fredrickson DS (1972) Estimations of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18:499–502
Diabetes Trial Unit. (2013). The Oxford Centre for Diabetes, Endocrinology and Metabolism [internet]. Oxford, UK: Oxford University; May 1. https://www.dtu.ox.ac.uk/homacalculator. Accessed 02 July 2020
Merino PM, Villarroel C, Jesam C, López P, Codner E (2017) New diagnostic criteria of polycystic ovarian morphology for adolescents: impact on prevalence and hormonal profile. Horm Res Paediatr 88:401–407. https://doi.org/10.1159/000481532
Bellver J, Rodríguez-Tabernero L, Robles A, Muñoz E, Martínez F, Landeras J et al (2018) Group of interest in Reproductive Endocrinology (GIER) of the Spanish fertility society (SEF). Polycystic ovary syndrome throughout a woman’s life. J Assist Reprod Genet 35:25–39. https://doi.org/10.1007/s10815-017-1047-7
Wang JG, Zhang Y, Chen HE, Li Y, Cheng XG, Xu L et al (2013) Comparison of two bioelectrical impedance analysis devices with dual energy X-ray absorptiometry and magnetic resonance imaging in the estimation of body composition. J Strength Condition Res 27:236–243. https://doi.org/10.1519/JSC.0b013e31824f2040
Bil E, Dilbaz B, Cirik DA, Ozelci R, Ozkaya E, Dilbaz S (2016) Metabolic syndrome and metabolic risk profile according to polycystic ovary syndrome phenotype. J Obstet Gynaecol Res 42:837–843. https://doi.org/10.1111/jog.12985
Legro RS, Arslanian SA, Ehrmann DA, Hoeger KM, Murad MH, Pasquali R, Welt CK, Endocrine Society (2013) Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 98:4565–4592. https://doi.org/10.1210/jc.2013-2350
Fernandes AR, de Sá Rosa e Silva AC, Romão GS, Pata MC, dos Reis RM (2005). Insulin resistance in adolescents with menstrual irregularities. J Pediatr Adolesc Gynecol 18:269-274. https://doi.org/10.1016/j.jpag.2005.05.006
Rosenfield RL (2015) The diagnosis of polycystic ovary syndrome in adolescents. Pediatrics 136:1154–1165. https://doi.org/10.1542/peds.2015-1430
Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, Janssen OE, Legro RS, Norman RJ, Taylor AE, Witchel SF, Androgen Excess Society (2006) Positions statement: criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an androgen excess society guideline. J Clin Endocrinol Metab 91:4237–4245. https://doi.org/10.1210/jc.2006-0178
Galobardes B, Davey Smith G, Jefferys M, McCarron P, Cohort GA (2005) Has acne increased? Prevalence of acne history among university students between 1948 and 1968. The Glasgow Alumni Cohort Study. Br J Dermatol 152:824–825. https://doi.org/10.1111/j.1365-2133.2005.06527.x
Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, Janssen OE, Legro RS, Norman RJ, Taylor AE, Witchel SF, Task Force on the Phenotype of the Polycystic Ovary Syndrome of The Androgen Excess and PCOS Society (2009) The androgen excess and PCOS society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril 91:456–488. https://doi.org/10.1016/j.fertnstert.2008.06.035
Fruzzetti F, Perini D, Lazzarini V, Parrini D, Genazzani AR (2009) Adolescent girls with polycystic ovary syndrome showing different phenotypes have a different metabolic profile associated with increasing androgen levels. Fertil Steril 92:626–634. https://doi.org/10.1016/j.fertnstert.2008.06.004
Goodman NF, Cobin RH, Futterweit W, Glueck JS, Legro RS, Carmina E (2015) American Association of clinical endocrinologists, American college of endocrinology, and androgen excess and PCOS society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome - part 2. Endocr Pract 21:1415–1426. https://doi.org/10.4158/EP15748.DSCPT2
Hickey M, Sloboda DM, Atkinson HC, Doherty DA, Franks S, Norman RJ, Newnham JP, Hart R (2009) The relationship between maternal and umbilical cord androgen levels and polycystic ovary syndrome in adolescence: a prospective cohort study. J Clin Endocrinol Metab 94:3714–3720. https://doi.org/10.1210/jc.2009-0544
Villarroel C, Merino PM, López P, Eyzaguirre FC, Van Velzen A, Iñiguez G, Codner E (2011) Polycystic ovarian morphology in adolescents with regular menstrual cycles is associated with elevated anti-Mullerian hormone. Hum Reprod 26:2861–2868. https://doi.org/10.1093/humrep/der223
Mortensen M, Rosenfield RL, Littlejohn E (2006) Functional significance of polycystic-size ovaries in healthy adolescents. J Clin Endocrinol Metab 91:3786–3790. https://doi.org/10.1210/jc.2006-0835
Dewailly D, Lujan ME, Carmina E, Cedars MI, Laven J, Norman RJ, Escobar-Morreale HF (2020) Definition and significance of polycystic ovarian morphology: a task force report from the Androgen excess and polycystic ovary syndrome society. Hum Reprod Update 20:334–352. https://doi.org/10.1093/humupd/dmt061
Sawathiparnich P, Weerakulwattana L, Santiprabhob J, Likitmaskul S (2005) Obese adolescent girls with polycystic ovary syndrome (PCOS) have more severe insulin resistance measured by HOMA-IR score than obese girls without PCOS. J Med Assoc Thai 88:S33–S37
Pasquali R, Gambineri A (2006) Polycystic ovary syndrome: a multifaceted disease from adolescence to adult age. Ann NY Acad Sci 1092:158–174. https://doi.org/10.1196/annals.1365.014
Leibel NI, Baumann EE, Kocherginsky M, Rosenfield RL (2006) Relationship of adolescent polycystic ovary syndrome to parental metabolic syndrome. J Clin Endocrinol Metab 91:1275–1283. https://doi.org/10.1210/jc.2005-1707
Arslanian SA, Lewy VD, Danadian K (2001) Glucose intolerance in obese adolescents with polycystic ovary syndrome: roles of insulin resistance and beta-cell dysfunction and risk of cardiovascular disease. J Clin Endocrinol Metab 86:66–71. https://doi.org/10.1210/jcem.86.1.7123
Legro RS, Kunselman AR, Dodson WC, Dunaif A (1999) Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J Clin Endocrinol Metab 84:165–169. https://doi.org/10.1210/jcem.84.1.5393
Ehrmann DA, Barnes RB, Rosenfield RL, Cavaghan MK, Imperial J (1999) Prevalence of impaired glucose tolerance and diabetes in women with polycystic ovary syndrome. Diabetes Care 22:141–146. https://doi.org/10.2337/diacare.22.1.141
Palmert MR, Gordon CM, Kartashov AI, Legro RS, Emans SJ, Dunaif A (2002) Screening for abnormal glucose tolerance in adolescents with polycystic ovary syndrome. J Clin Endocrinol Metab 87:1017–1023. https://doi.org/10.1210/jcem.87.3.8305
Alemzadeh R, Kichler J, Calhoun M (2010) Spectrum of metabolic dysfunction in relationship with hyperandrogenemia in obese adolescent girls with polycystic ovary syndrome. Eur J Endocrinol 162:1093–1099. https://doi.org/10.1530/EJE-10-0205
Legro RS, Kunselman AR, Dunaif A (2001) Prevalence and predictors of dyslipidemia in women with polycystic ovary syndrome. Am J Med 111:607–613. https://doi.org/10.1016/s0002-9343(01)00948-2
Demirel F, Bideci A, Cinaz P, Camurdan MO, Biberoğlu G, Yesilkaya E, Hasanoğlu A (2007) Serum leptin, oxidized low density lipoprotein and plasma asymmetric dimethylarginine levels and their relationship with dyslipidaemia in adolescent girls with polycystic ovary syndrome. Clin Endocrinol 67:129–134. https://doi.org/10.1111/j.1365-2265.2007.02849.x
Pedroso DC, Melo AS, Carolo AL, Vieira CS, Rosa e Silva AC, dos Reis RM, (2012) Frequency and risk factors for metabolic syndrome in adolescents and adults women with polycystic ovary syndrome. Rev Bras Ginecol Obstet 34:357–361. https://doi.org/10.1590/s0100-72032012000800003
Vural B, Caliskan E, Turkoz E, Kilic T, Demirci A (2005) Evaluation of metabolic syndrome frequency and premature carotid atherosclerosis in young women with polycystic ovary syndrome. Hum Reprod 20:2409–2413. https://doi.org/10.1093/humrep/dei100
Salley KE, Wickham EP, Cheang KI, Essah PA, Karjane NW, Nestler JE (2007) Glucose intolerance in polycystic ovary syndrome–a position statement of the Androgen Excess Society. J Clin Endocrinol Metab 92:4546–4556. https://doi.org/10.1210/jc.2007-1549
Park HR, Choi Y, Lee HJ, Oh JY, Hong YS, Sung YA (2007) The metabolic syndrome in young Korean women with polycystic ovary syndrome. Diabetes Res Clin Pract 77:S243–S246. https://doi.org/10.1016/j.diabres.2007.01.065
Han Y, Kim HS, Hye-Jin L, Jee-Young O, Yeon-Ah S (2015) Metabolic effects of polycystic ovary syndrome in adolescents. Ann Pediatr Endocrinol Metab 20:136–142. https://doi.org/10.6065/apem.2015.20.3.136
Acknowledgements
The authors are grateful to Liberty Medical Communications, LLC, for English proofreading.
Funding
This research did not receive any specific grant from any funding agency.
Author information
Authors and Affiliations
Contributions
SFdM: design, data description, statistical analysis, and writing the manuscript. MASdM: data search, revision of the manuscript. BBB: data search, data analysis, revision of the manuscript. MMWY: data search, revision of the manuscript. GARM: data search, revision of the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors fully declare there is either no financial or other conflicts of interest that could be perceived as prejudicing the impartiality of this study.
Ethics approval
A specific signed informed consent document approved by the local Committee for Ethics in Research was not necessary at this time because only the medical records of all patients were reviewed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
de Medeiros, S.F., de Medeiros, M.A.S., Barbosa, B.B. et al. Comparison of metabolic and obesity biomarkers between adolescent and adult women with polycystic ovary syndrome. Arch Gynecol Obstet 303, 739–749 (2021). https://doi.org/10.1007/s00404-020-05867-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-020-05867-x