Abstract
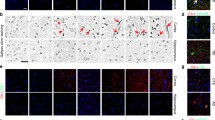
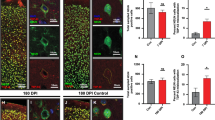
The pathologic phosphorylation and sub-cellular translocation of neuronal transactive response-DNA binding protein (TDP-43) was identified as the major disease protein in frontotemporal lobar degeneration (FTLD) with ubiquitinated inclusions, now termed FTLD-TDP, and amyotrophic lateral sclerosis (ALS). More recently, TDP-43 proteinopathy has been reported in dementia pugilistica or chronic traumatic encephalopathy caused by repetitive traumatic brain injury (TBI). While a single TBI has been linked to the development of Alzheimer’s disease and an increased frequency of neurofibrillary tangles, TDP-43 proteinopathy has not been examined with survival following a single TBI. Using immunohistochemistry specific for both pathological phosphorylated TDP-43 (p-TDP-43) and phosphorylation-independent TDP-43 (pi-TDP-43), we examined acute (n = 23: Survival < 2 weeks) and long-term (n = 39; 1–47 years survival) survivors of a single TBI versus age-matched controls (n = 47). Multiple regions were examined including the hippocampus, medial temporal lobe, cingulate gyrus, superior frontal gyrus and brainstem. No association was found between a history of single TBI and abnormally phosphorylated TDP-43 (p-TDP-43) inclusions. Specifically, just 3 of 62 TBI cases displayed p-TDP-43 pathology versus 2 of 47 control cases. However, while aggregates of p-TDP-43 were not increased acutely or long-term following TBI, immunoreactivity to phosphorylation-independent TDP-43 was commonly increased in the cytoplasm following TBI with both acute and long-term survival. Moreover, while single TBI can induce multiple long-term neurodegenerative changes, the absence of TDP-43 proteinopathy may indicate a fundamental difference in the processes induced following single TBI from those of repetitive TBI.



Similar content being viewed by others
References
Abhyankar MM, Urekar C, Reddi PP (2007) A novel CpG-free vertebrate insulator silences the testis-specific SP-10 gene in somatic tissues: role for TDP-43 in insulator function. J Biol Chem 282:36143–36154
Acharya KK, Govind CK, Shore AN, Stoler MH, Reddi PP (2006) cis-Requirement for the maintenance of round spermatid-specific transcription. Dev Biol 295:781–790
Adams JH, Graham DI, Murray LS, Scott G (1982) Diffuse axonal injury due to nonmissile head injury in humans: an analysis of 45 cases. Ann Neurol 12:557–563
Amador-Ortiz C, Lin WL, Ahmed Z et al (2007) TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer’s disease. Ann Neurol 61:435–445
Arai T, Mackenzie IR, Hasegawa M et al (2009) Phosphorylated TDP-43 in Alzheimer’s disease and dementia with Lewy bodies. Acta Neuropathol 117:125–136
Ayala YM, Pagani F, Baralle FE (2006) TDP43 depletion rescues aberrant CFTR exon 9 skipping. FEBS Lett 580:1339–1344
Blumbergs PC, Scott G, Manavis J et al (1994) Staining of amyloid precursor protein to study axonal damage in mild head injury. Lancet 344:1055–1056
Buratti E, Baralle FE (2001) Characterization and functional implications of the RNA binding properties of nuclear factor TDP-43, a novel splicing regulator of CFTR exon 9. J Biol Chem 276:36337–36343
Buratti E, Baralle FE (2008) Multiple roles of TDP-43 in gene expression, splicing regulation, and human disease. Front Biosci 13:867–878
Buratti E, Brindisi A, Pagani F, Baralle FE (2004) Nuclear factor TDP-43 binds to the polymorphic TG repeats in CFTR intron 8 and causes skipping of exon 9: a functional link with disease penetrance. Am J Hum Genet 74:1322–1325
Buratti E, Dork T, Zuccato E et al (2001) Nuclear factor TDP-43 and SR proteins promote in vitro and in vivo CFTR exon 9 skipping. EMBO J 20:1774–1784
Chen XH, Johnson VE, Uryu K, Trojanowski JQ, Smith DH (2009) A lack of amyloid beta plaques despite persistent accumulation of amyloid beta in axons of long-term survivors of traumatic brain injury. Brain Pathol 19:214–223
Chen XH, Siman R, Iwata A et al (2004) Long-term accumulation of amyloid-beta, beta-secretase, presenilin-1, and caspase-3 in damaged axons following brain trauma. Am J Pathol 165:357–371
Chen-Plotkin AS, Lee VM, Trojanowski JQ (2010) TAR DNA-binding protein 43 in neurodegenerative disease. Nat Rev Neurol 6:211–220
Corsellis JA, Bruton CJ, Freeman-Browne D (1973) The aftermath of boxing. Psychol Med 3:270–303
Dale GE, Leigh PN, Luthert P, Anderton BH, Roberts GW (1991) Neurofibrillary tangles in dementia pugilistica are ubiquitinated. J Neurol Neurosurg Psychiatry 54:116–118
Fleminger S, Oliver DL, Lovestone S, Rabe-Hesketh S, Giora A (2003) Head injury as a risk factor for Alzheimer’s disease: the evidence 10 years on; a partial replication. J Neurol Neurosurg Psychiatry 74:857–862
Geddes JF, Vowles GH, Beer TW, Ellison DW (1997) The diagnosis of diffuse axonal injury: implications for forensic practice. Neuropathol Appl Neurobiol 23:339–347
Geddes JF, Vowles GH, Nicoll JA, Revesz T (1999) Neuronal cytoskeletal changes are an early consequence of repetitive head injury. Acta Neuropathol 98:171–178
Geddes JF, Whitwell HL, Graham DI (2000) Traumatic axonal injury: practical issues for diagnosis in medicolegal cases. Neuropathol Appl Neurobiol 26:105–116
Gedye A, Beattie BL, Tuokko H, Horton A, Korsarek E (1989) Severe head injury hastens age of onset of Alzheimer’s disease. J Am Geriatr Soc 37:970–973
Geser F, Martinez-Lage M, Kwong LK, Lee VM, Trojanowski JQ (2009) Amyotrophic lateral sclerosis, frontotemporal dementia and beyond: the TDP-43 diseases. J Neurol 256:1205–1214
Geser F, Martinez-Lage M, Robinson J et al (2009) Clinical and pathological continuum of multisystem TDP-43 proteinopathies. Arch Neurol 66:180–189
Geser F, Robinson JL, Malunda JA et al (2010) Pathological 43-kDa transactivation response DNA-binding protein in older adults with and without severe mental illness. Arch Neurol 67:1238–1250
Graham DI, Gentleman SM, Lynch A, Roberts GW (1995) Distribution of beta-amyloid protein in the brain following severe head injury. Neuropathol Appl Neurobiol 21:27–34
Graves AB, White E, Koepsell TD et al (1990) The association between head trauma and Alzheimer’s disease. Am J Epidemiol 131:491–501
Guo Z, Cupples LA, Kurz A et al (2000) Head injury and the risk of AD in the MIRAGE study. Neurology. 54:1316–1323
Hasegawa M, Arai T, Nonaka T et al (2008) Phosphorylated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Ann Neurol 64:60–70
Higashi S, Iseki E, Yamamoto R et al (2007) Concurrence of TDP-43, tau and alpha-synuclein pathology in brains of Alzheimer’s disease and dementia with Lewy bodies. Brain Res 1184:284–294
Hu WT, Josephs KA, Knopman DS et al (2008) Temporal lobar predominance of TDP-43 neuronal cytoplasmic inclusions in Alzheimer disease. Acta Neuropathol 116:215–220
Huber AGK, Kelemen J, Cervod-Navarro J (1993) Density of amyloid plaques in brains after head trauma. J Neurotrauma 10(Suppl):S180
Igaz LM, Kwong LK, Xu Y et al (2008) Enrichment of C-terminal fragments in TAR DNA-binding protein-43 cytoplasmic inclusions in brain but not in spinal cord of frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Am J Pathol 173:182–194
Ikonomovic MD, Uryu K, Abrahamson EE et al (2004) Alzheimer’s pathology in human temporal cortex surgically excised after severe brain injury. Exp Neurol 190:192–203
Inukai Y, Nonaka T, Arai T et al (2008) Abnormal phosphorylation of Ser409/410 of TDP-43 in FTLD-U and ALS. FEBS Lett 582:2899–2904
Johnson VE, Stewart W, Smith DH (2010) Traumatic brain injury and amyloid-beta pathology: a link to Alzheimer’s disease? Nat Rev Neurosci. 11:361–370
Johnson VE, Stewart W, Smith DH (2011) Amyloid and tau pathologies many years following single traumatic brain injury in humans. Brain Pathol (in press)
Kanazawa M, Kakita A, Igarashi H et al (2011) Biochemical and histopathological alterations in TAR DNA-binding protein-43 after acute ischemic stroke in rats. J Neurochem 116:957–965
King A, Sweeney F, Bodi I et al (2010) Abnormal TDP-43 expression is identified in the neocortex in cases of dementia pugilistica, but is mainly confined to the limbic system when identified in high and moderate stages of Alzheimer’s disease. Neuropathology 30:408–419
Kwong LK, Uryu K, Trojanowski JQ, Lee VM (2008) TDP-43 proteinopathies: neurodegenerative protein misfolding diseases without amyloidosis. Neurosignals 16:41–51
Lee EB, Lee VM, Trojanowski JQ, Neumann M (2008) TDP-43 immunoreactivity in anoxic, ischemic and neoplastic lesions of the central nervous system. Acta Neuropathol 115:305–311
Lee EB, Lee VM-Y, Trojanowski JQ (2011) Gains or losses: molecular mechanisms of TDP-43 mediated neurodegeneration. Nat Rev Neurosci (in press)
McDonald KK, Aulas A, Destroismaisons L et al (2011) TAR DNA-binding protein 43 (TDP-43) regulates stress granule dynamics via differential regulation of G3BP and TIA-1. Hum Mol Genet 20:1400–1410
McKee AC, Cantu RC, Nowinski CJ et al (2009) Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol 68:709–735
McKee AC, Gavett BE, Stern RA et al (2010) TDP-43 proteinopathy and motor neuron disease in chronic traumatic encephalopathy. J Neuropathol Exp Neurol 69:918–929
Mercado PA, Ayala YM, Romano M, Buratti E, Baralle FE (2005) Depletion of TDP 43 overrides the need for exonic and intronic splicing enhancers in the human apoA-II gene. Nucleic Acids Res 33:6000–6010
Moisse K, Mepham J, Volkening K et al (2009) Cytosolic TDP-43 expression following axotomy is associated with caspase 3 activation in NFL-/- mice: support for a role for TDP-43 in the physiological response to neuronal injury. Brain Res 1296:176–186
Moisse K, Volkening K, Leystra-Lantz C et al (2009) Divergent patterns of cytosolic TDP-43 and neuronal progranulin expression following axotomy: implications for TDP-43 in the physiological response to neuronal injury. Brain Res 1249:202–211
Molgaard CA, Stanford EP, Morton DJ et al (1990) Epidemiology of head trauma and neurocognitive impairment in a multi-ethnic population. Neuroepidemiology 9:233–242
Mortimer JA, French LR, Hutton JT, Schuman LM (1985) Head injury as a risk factor for Alzheimer’s disease. Neurology 35:264–267
Mortimer JA, van Duijn CM, Chandra V et al (1991) Head trauma as a risk factor for Alzheimer’s disease: a collaborative re-analysis of case–control studies. EURODEM Risk Factors Research Group. Int J Epidemiol 20(Suppl 2):S28–S35
Nakashima-Yasuda H, Uryu K, Robinson J et al (2007) Co-morbidity of TDP-43 proteinopathy in Lewy body related diseases. Acta Neuropathol 114:221–229
Nemetz PN, Leibson C, Naessens JM et al (1999) Traumatic brain injury and time to onset of Alzheimer’s disease: a population-based study. Am J Epidemiol 149:32–40
Neumann M, Kwong LK, Lee EB et al (2009) Phosphorylation of S409/410 of TDP-43 is a consistent feature in all sporadic and familial forms of TDP-43 proteinopathies. Acta Neuropathol 117:137–149
Neumann M, Kwong LK, Sampathu DM, Trojanowski JQ, Lee VM (2007) TDP-43 proteinopathy in frontotemporal lobar degeneration and amyotrophic lateral sclerosis: protein misfolding diseases without amyloidosis. Arch Neurol 64:1388–1394
Neumann M, Sampathu DM, Kwong LK et al (2006) Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 314:130–133
O’Meara ES, Kukull WA, Sheppard L et al (1997) Head injury and risk of Alzheimer’s disease by apolipoprotein E genotype. Am J Epidemiol 146:373–384
Omalu BI, DeKosky ST, Hamilton RL et al (2006) Chronic traumatic encephalopathy in a national football league player: part II. Neurosurgery. 59:1086–1092 (discussion 1092–1083)
Omalu BI, DeKosky ST, Minster RL et al (2005) Chronic traumatic encephalopathy in a National Football League player. Neurosurgery 57:128–134 (discussion 128–134)
Ou SH, Wu F, Harrich D, Garcia-Martinez LF, Gaynor RB (1995) Cloning and characterization of a novel cellular protein, TDP-43, that binds to human immunodeficiency virus type 1 TAR DNA sequence motifs. J Virol 69:3584–3596
Plassman BL, Havlik RJ, Steffens DC et al (2000) Documented head injury in early adulthood and risk of Alzheimer’s disease and other dementias. Neurology 55:1158–1166
Roberts GW, Allsop D, Bruton C (1990) The occult aftermath of boxing. J Neurol Neurosurg Psychiatry 53:373–378
Roberts GW, Gentleman SM, Lynch A, Graham DI (1991) beta A4 amyloid protein deposition in brain after head trauma. Lancet 338:1422–1423
Roberts GW, Gentleman SM, Lynch A et al (1994) Beta amyloid protein deposition in the brain after severe head injury: implications for the pathogenesis of Alzheimer’s disease. J Neurol Neurosurg Psychiatry 57:419–425
Salib E, Hillier V (1997) Head injury and the risk of Alzheimer’s disease: a case control study. Int J Geriatr Psychiatry. 12:363–368
Sato T, Takeuchi S, Saito A et al (2009) Axonal ligation induces transient redistribution of TDP-43 in brainstem motor neurons. Neuroscience. 164:1565–1578
Schofield PW, Tang M, Marder K et al (1997) Alzheimer’s disease after remote head injury: an incidence study. J Neurol Neurosurg Psychiatry 62:119–124
Schwab C, Arai T, Hasegawa M, Yu S, McGeer PL (2008) Colocalization of transactivation-responsive DNA-binding protein 43 and huntingtin in inclusions of Huntington disease. J Neuropathol Exp Neurol 67:1159–1165
Smith DH, Chen XH, Iwata A, Graham DI (2003) Amyloid beta accumulation in axons after traumatic brain injury in humans. J Neurosurg 98:1072–1077
Smith DH, Chen XH, Nonaka M et al (1999) Accumulation of amyloid beta and tau and the formation of neurofilament inclusions following diffuse brain injury in the pig. J Neuropathol Exp Neurol 58:982–992
Sullivan P, Petitti D, Barbaccia J (1987) Head trauma and age of onset of dementia of the Alzheimer type. JAMA. 257:2289–2290
Tokuda T, Ikeda S, Yanagisawa N, Ihara Y, Glenner GG (1991) Re-examination of ex-boxers’ brains using immunohistochemistry with antibodies to amyloid beta-protein and tau protein. Acta Neuropathol 82:280–285
Uryu K, Chen XH, Martinez D et al (2007) Multiple proteins implicated in neurodegenerative diseases accumulate in axons after brain trauma in humans. Exp Neurol 208:185–192
Uryu K, Nakashima-Yasuda H, Forman MS et al (2008) Concomitant TAR-DNA-binding protein 43 pathology is present in Alzheimer disease and corticobasal degeneration but not in other tauopathies. J Neuropathol Exp Neurol 67:555–564
Wang HY, Wang IF, Bose J, Shen CK (2004) Structural diversity and functional implications of the eukaryotic TDP gene family. Genomics. 83:130–139
Wang IF, Wu LS, Shen CK (2008) TDP-43: an emerging new player in neurodegenerative diseases. Trends Mol Med. 14:479–485
Acknowledgments
This work was supported by National Institutes of Health grants NS038104, AG10124, AG17546 and NS056202. We would also like to thank Ms. Janice E. Stewart for her technical assistance with immunohistochemical staining procedures.
Conflict of interest
None of the authors have any conflict of Interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Johnson, V.E., Stewart, W., Trojanowski, J.Q. et al. Acute and chronically increased immunoreactivity to phosphorylation-independent but not pathological TDP-43 after a single traumatic brain injury in humans. Acta Neuropathol 122, 715–726 (2011). https://doi.org/10.1007/s00401-011-0909-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-011-0909-9