Abstract
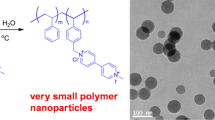
Nanoparticle colloids of methoxy poly(ethylene glycol)-b-poly(D,L-lactide) (MPEG-b-PDLL) diblock copolymer were prepared by a modified spontaneous emulsification solvent diffusion method using acetone/ethanol as the mixture organic solvents. The MPEG-b-PDLL was synthesized by ring-opening polymerization of D,L-lactide using stannous octoate and MPEG with molecular weight of 5,000 g/mol as the initiating system. The MPEG-b-PDLL obtained was an amorphous polymer with molecular weight of 73,600 g/mol. Influences of acetone/ethanol (v/v) ratios and Tween 80 surfactant concentrations on characteristics of the colloidal nanoparticles were investigated and discussed. Light-scattering analysis showed that average diameters of the surfactant-free colloidal nanoparticles were in the range of 86–124 nm. The nanoparticle sizes decreased as the ethanol ratio increased. The Tween 80 did not show the significant effect on the nanoparticle sizes. Scanning electron micrographs of dried nanoparticles that demonstrated the aggregation of most particles suggested they were the soft nanoparticles. However, the dried nanoparticle morphology can be observed from scanning electron microscopy as having a spherical shape and smooth surfaces.




Similar content being viewed by others
References
Lucke A, Tebmar J, Schnell E, Schmeer G, Gopferich A (2000) Biomaterials 21:2361
Sun J, Hong Z, Yang L, Tang Z, Chen X, Jing X (2004) Polymer 45:5969
Stefani M, Coudane J, Vert M (2006) Polym Degrad Stab 91:2554
Kim SY, Shin IG, Lee YM (1998) J Control Release 56:197
Kim SY, Lee YM, Kang JS (2005) J Biomed Mater Res 74A:581
Pierri E, Avgoustakis K (2005) J Biomed Mater Res 75A:639
Faria TJ, Campos AM, Senna EL (2005) Macromol Symp 229:228
Riley T, Heald CR, Stolnik S, Garnett MC, Illum L, Davis SS (2003) Langmuir 19:8428
Stolnik S, Illum L, Davis SS (1995) Adv Drug Deliv Rev 16:195
Stolnik S, Heald CR, Neal J, Garnett MC, Davis SS, Illum L, Purkiss SC, Barlow RJ, Gellert PR (2001) J Drug Target 9:361
Gorner T, Gref R, Michenot D, Sommer F, Tran MN, Dellacherie E (1999) J Control Release 57:259
Belbella A, Vauthier C, Fessi H, Devissaguet J-P, Puisieux F (1996) Int J Pharm 129:95
Hu Y, Jiang X, Ding Y, Zhang L, Yang C, Zhang J, Chen J, Yang Y (2003) Biomaterials 24:2395
Allemann E, Leroux JC, Gurnay R, Doelker E (1993) Pharm Res 10:1732
Dai Z, Piao L, Zhang X, Deng M, Chen X, Jing X (2004) Colloid Polym Sci 282:343
Murakami H, Kobayashi M, Takeuchi H, Kawashima Y (1999) Int J Pharm 187:143
Niwa T, Takeuchi H, Hino T, Kunou N, Kawashima Y (1993) J Control Release 25:89
Magenheim B, Benita S (1991) STP Pharm Sci 1:221
Quintanar-Guerrero D, Fessi H, Allemann E, Doelker E (1996) Int J Pharm 143:133
Acknowledgements
The authors would like to acknowledge the Faculty of Science, Mahasarakham University, and the Thailand Research Fund (TRF) for financial support (DBG4880008). The authors would like to thank Mr. Teerasak Kerdchan from the Faculty of Pharmacy, Mahasarakham University, Mahasarakham, Thailand, for light-scattering particle size analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baimark, Y., Srisa-ard, M., Threeprom, J. et al. Preparation of nanoparticle colloids of methoxy poly(ethylene glycol)-b-poly(D,L-lactide): effects of surfactant and organic solvent. Colloid Polym Sci 285, 1521–1525 (2007). https://doi.org/10.1007/s00396-007-1731-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-007-1731-8