Abstract
Background
Since 1993, data on the care and quality of life of patients with inflammatory rheumatic diseases have been collected in the German National Database (NDB) of the regional collaborative rheumatology centers.
Objective
In this review long-term trends on treatment, disease activity and gainful employment of the most common inflammatory rheumatic diseases are presented and the most important analyses from 25 years of the NDB are summarized.
Methods
Between 15 and 17 rheumatological institutions take part in the NDB and once a year collect data from a total of more than 10,000 patients. The rheumatologists document the disease status and care, the patients report on their state of health and the effects of the disease.
Results
The biologics era at the beginning of the twenty-first century has led to changes in the therapeutic spectrum of most inflammatory rheumatic diseases, especially in rheumatoid arthritis and ankylosing spondylitis. Some basic therapies formerly used are hardly used anymore and glucocorticoids are used less frequently. Methotrexate has remained the standard therapy for rheumatoid arthritis over the years. Nowadays, nearly 30% of patients with rheumatoid arthritis receive treatment with biologics. Disease activity, functional and social restrictions have decreased across all diseases.
Conclusion
The improved health status of many patients with rheumatic diseases confirms the high level of care provided by the rheumatism centers involved in the NDB. The increasing specification of measuring instruments and the standardization of documentation systems are major challenges that the NDB will have to face in the coming years if it is to remain in the digital age.
Zusammenfassung
Hintergrund
Seit 1993 werden in der Kerndokumentation der Regionalen Kooperativen Rheumazentren Daten zur Versorgung und Lebensqualität von Patienten mit entzündlich rheumatischen Erkrankungen erhoben.
Fragestellung
In dieser Übersichtsarbeit werden Langzeittrends zu Therapien, Krankheitsaktivität und Erwerbstätigkeit der häufigsten entzündlich rheumatischen Erkrankungen vorgestellt und die wichtigsten Analysen aus 25 Jahren Kerndokumentation zusammengefasst.
Methoden
Zwischen 15 und 17 rheumatologische Einrichtungen nehmen an der Kerndokumentation teil und erfassen 1‑mal jährlich Daten von insgesamt mehr als 10.000 Patienten. Die Rheumatologen dokumentieren den Krankheitsstatus und die Versorgung, die Patienten berichten über ihren Gesundheitszustand und die Auswirkungen der Erkrankung.
Ergebnisse
Der Beginn der Biologikaära Anfang des 21. Jahrhunderts hat das Therapiespektrum insbesondere der rheumatoiden Arthritis und ankylosierenden Spondylitis verändert. Einige früher häufig verwendete Basistherapeutika werden heute kaum noch eingesetzt, Glukokortikoide werden seltener gebraucht. Bei der rheumatoiden Arthritis hat sich Methotrexat über die Jahre hinweg als Standardtherapie etabliert, knapp 30 % der Patienten mit rheumatoider Arthritis erhalten heute eine Biologikatherapie. Krankheitsaktivität, funktionelle und soziale Einschränkungen sind krankheitsübergreifend zurückgegangen.
Schlussfolgerungen
Der verbesserte Gesundheitsstatus vieler Rheumaerkrankter bestätigt das hohe Versorgungsniveau innerhalb der an der Kerndokumentation beteiligten Rheumazentren. Die zunehmende Spezifizierung der Messinstrumente und die Vereinheitlichung der Dokumentationssysteme sind wesentliche Herausforderungen, der sich die Kerndokumentation in den nächsten Jahren stellen muss, um im digitalen Zeitalter bestehen zu bleiben.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The German national database (NDB) was established in 1993 as an instrument for monitoring the health care of inflammatory rheumatic diseases. A pilot project took place in 1992 at several outpatient clinics and rheumatological practices in Berlin. With the support of the German Federal Ministry of Health, the documentation could be expanded to a nationwide collection in 22 rheumatism centers. Data from 25,000 patients with inflammatory rheumatic diseases from 1998 were already analyzed in the first publications [1,2,3]. From 1997 to 2006, the NDB was funded by the Federal Ministry of Research and Technology within the framework of the Competence Network Rheumatism. With the expiration of federal funding, it was possible to achieve follow-up funding from 2007, which is jointly supported by the Association of Regional Cooperative Rheumatology Centers and various member companies in the Association of Corporate Members of the German Society for Rheumatology [4]. The NDB has been continued in this form for 13 years now. Community funding guarantees the German Rheumatism Research Centre (Deutsches Rheuma-Forschungszentrum, DRFZ) full academic freedom in the implementation of the project and the analysis and publication of the results. In the 25 years to date, more than 260,000 patients with inflammatory rheumatic diseases have been documented. Numerous national and international publications have emerged from the data. Key findings include trend analyses of care and burden of diseases [5,6,7,8], employment and cost analyses [9,10,11,12,13].
All inflammatory rheumatic disease patterns are recorded in the NDB. These include rheumatoid arthritis (RA), ankylosing spondylitis (AS), psoriatic arthritis (PsA), systemic lupus erythematosus (SLE) and other collagenoses and vasculitides. There are also a handful of patients documented with rare diseases, even within rheumatology, e.g. cryoglobulinemia, brucellosis, thrombangiitis obliterans or Felty syndrome. However, since not every rheumatological disease can be recorded with specifically developed scores, the documentation focuses on the most common disease, RA [4].
The NDB is a long-term observational study which, in addition to clinical studies and registry data, is justified in mapping daily routine care within internal rheumatology. In this review, which was produced in the year of the 25th anniversary of the NDB, current results from the analyses compiled annually for the participating institutions are reported and the essential studies of the past decades summarized.
Patients
In the NDB, patients are documented in the participating institutions once a year—if possible consecutively. All inflammatory rheumatic diseases can be recorded on suspicion or confirmed diagnosis. About half of all diagnoses are RA, while 10% of patients have AS, 10% PsA and about 6% SLE. The remaining cases are distributed among other arthritides, collagenoses and vasculitides. Patients can, but do not have to, be documented in the course of their treatment. For the interpretation of the data it is important that cross-sectional data from the individual years is presented, i.e. for each year all patients documented in that year are reported. The annual number of cases is declining (~13,000), this is mainly due to the number of participating institutions, which fell significantly in 2005 with the switch from the paper version to a computer-assisted input and, since then, has decreased to 15–17 institutions. The quality of the documented data has increased with the conversion to electronic documentation. The NDB is integrated into various documentation systems. Their use is not available in all facilities and makes it difficult to attract new participating centers. Table 1 shows the number of documented cases for RA, AS, PsA and SLE from 1996 to 2016.
Epidemiological trends
On the basis of the NDB, dynamics in the epidemiology of rheumatological diseases can be determined. In RA, and especially in AS and PsA, an increase in the age of onset of the disease over time can be observed (Fig. 1). In the case of RA, this applies in particular to seronegative patients (∆ 6 years). The age of onset does not show any change in patients with SLE. The reason for these observations is not known as yet. The proportion of women is constant in RA (74–75%), PsA (52–55%) and SLE (88–90%), while it has risen from 30% to 38% in AS. The proportion of rheumatoid factor-positive patients has not changed substantially (61–70% in the years 2009–2016); the proportion of smokers has also remained similar since 2005 (52% in 2005, 54% in 2016). The proportion of patients with a higher level of education has increased significantly (16% with over 13 years of education in 1993, 31% in 2016). This corresponds to an overall higher level of educational attainment among the population. The mean age in all diseases has increased over time (Table 1). This is mainly due to the fact that less newly referred but more patients with long disease duration are recorded.
Increase in the age of disease onset, comparison 1990s and 2000s Patients with a symptom duration of <5 years (RA, SLE, PsA) or <10 years (AS) were considered. Reference lines show the median. The difference in the median age of onset for the two periods 1994–1996 and 2014–2016 was 3 years (RA), 5.5 years (AS), 6 years (PsA) and 0 years (SLE). RA rheumatoid arthritis, AS ankylosing spondylitis, PsA psoriatic arthritis, SLE systemic lupus erythematosus
Developments in rheumatological care
The proportion of patients that visit a rheumatologist within 12 months of the onset of symptoms has increased in all diseases. However, it is still low for AS at 45% in 2016 (RA: 73%, SLE: 71%, PsA: 66%). The mean disease duration at the first contact with a rheumatologist has decreased (median 1994/2016: 1.0/0.5 years [RA], 5.0/2.1 years [AS], 2.0/0.6 years [PsA]). But here, too, AS has the highest proportion of patients that have been ill for several years before seeing a rheumatologist.
Treatment changes
The most significant changes in drug therapy have occurred with the introduction of biological disease-modifying antirheumatic drugs (bDMARDs). In 2001, the first patients were enrolled with bDMARDs, and by 2016 the proportion had increased to 28% (RA), 33% (PsA) and 50% (AS) (Fig. 2). In RA and PsA, more than half of bDMARD therapies are combined with a conventional synthetic (cs) DMARD (60%), most commonly with methotrexate (MTX). In AS, bDMARDs are predominantly administered as monotherapy (84%). Etanercept and adalimumab are the most commonly used substances, followed by rituximab and tocilizumab (RA) or golimumab (AS, PsA). MTX is the most commonly used csDMARD as a mono- and combination therapy. MTX and sulfasalazine are used less frequently in AS. The use of glucocorticoids decreased slightly in 2016, with 45% (RA), 10% (AS), 18% (PsA) and 62% (SLE) receiving glucocorticoids. At the same time, the proportion of patients treated at doses above 7.5 mg prednisolone equivalent per day decreased from 28% (1996) to 12% (2016) for RA, from 26 to 14% for PsA and from 38 to 13% for SLE. Nonsteroidal anti-inflammatory drugs (NSAIDs) are also on the decline, with 25% (RA) to 45% (AS) receiving NSAIDs in 2016. The use of analgesics has increased (18% AS, 20% RA in 2016).
Osteoporosis prevention and treatment take higher priority today
In collagenoses and vasculitides, the development of biologics has not yet led to pronounced changes in the therapy spectrum. In 2016 less than 10% of all patients with collagenoses or vasculitides were treated with biologics. Antimalarials were used in 67% of all SLE patients in 2016. Mycofenolate mofetil (13%) has been established as an alternative to azathioprine.
Today, osteoporosis prophylaxis and therapy have gained more importance across all diseases and are used in 47% (RA), 27% (AS), 31% (PsA) and 55% (SLE). Physiotherapy has been used slightly more frequently as non-drug therapy since 2008, while occupational therapy, rheumatic functional training and patient education have all been recorded in less than 5% of patients. Joint replacement due to RA is continuously declining (5% in 1996, 2% in 2016).
Decrease in disease activity and severity
The proportion of RA patients that are classified by rheumatologists as having a high degree of severity has fallen from 23% (1994) to 13% (2016). The mean disease activity score 28 (DAS28) decreased from 4.6 (1997) to 3.1 (2016). The proportion of patients in remission (DAS28 < 2.6) increased during this period from 13 to 39%, especially in patients with long disease duration. The proportion of patients classified by rheumatologists as having low disease activity (0–3 on a numeric rating scale (NRS) of 0–10) increased from 49 (1996) to 84% (2016).
The proportion of AS patients with inactive/moderate disease (ankylosing spondylitis disease activity score (ASDAS) < 2.1) increased from 39% (2011) to 47% (2016). The average Bath ankylosing spondylitis disease activity index (BASDAI) fell from 4.2 (2005) to 3.5 (2016). Further disease-specific activity or functional markers are not collected in the NDB or are only collected in a small number of cases. The systemic lupus erythematosus disease activity index (SLEDAI) has been included in the documentation of SLE patients since 2005, but is only documented for 1–12% of cases (N = 6–101 patients). The disease activity index for psoriatic arthritis (DAPSA) was calculated retrospectively for PsA. This requires 66/68 joint counts, which were only recorded in the specific PsA module from 2007 for about a quarter of all PsA patients. As this group differs from the overall collective in terms of age structure and therapy, the authors cannot make any statement on changes in the proportions of remission in SLE and PsA.
Patient-reported parameters
Functional status has significantly improved. The mean Hannover functional ability questionnaire (FFbH; function of 0–100%) in RA patients increased from 67 (1994) to 74 (2016). The mean FFbH is 80 in patients with a disease duration of <10 years. The proportion of patients with impaired functional status (FFbH < 70) decreased from 53 to 36%. The mean Bath ankylosing spondylitis functional index (BASFI) in patients with AS decreased from 4.2 (2005) to 3.6 (2016).
The proportion of patients reporting severe pain (7–10 on a NRS of 0–10) decreased from 33% (1993) to 16% (2016) for RA, from 32 to 19% for AS, from 26 to 16% for PsA and from 17 to 12% for SLE.
Decline in hospital stays and increase in gainful employment
Inpatient stays are declining and occurred in ≤10% of all patients in 2016. The average duration of stays has fallen substantially across all diseases (Table 2).
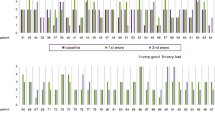
The proportion of employment at working age (<65 years) has risen steadily (Table 2). This applies to all four disease groups, men and women, as well as younger and older patients. Incapacity to work due to rheumatic diseases is also declining strongly, accompanied by a decrease in sick leave in days (Table 2; Fig. 3). Between 1996 and 2016, for example, the average number of absentee days for all gainfully employed RA patients fell from 30 to 9, despite higher labor force participation. In the other reported rheumatic diseases, absenteeism due to illness has also fallen to around one third.
Decline in incapacity to work among employed patients: a comparison of 1997 and 2016. The box plots show the median (line in the box), the 25–75th percentile (boundaries of the box) and the 10–90th percentile (boundaries of the thin lines above and below the box). RA rheumatoid arthritis, AS ankylosing spondylitis, PsA psoriatic arthritis, SLE systemic lupus erythematosus
Discussion
The idea of establishing a long-term monitoring system for internal rheumatological care with the NDB more than 25 years ago has led to a very successful and nationally and internationally known project. Due to the high willingness of the participating rheumatologists and patients to document information on the disease status over many years, a unique database could be established. With a high continuity of the measuring instruments, it reflects long-term trends over time with regard to therapeutic care, disease activity and disease burden of those affected.
Changes in age at disease onset, in health behavior and also in educational status are variables of epidemiological relevance when historical and current data are compared. Analyses of differences in disease activity in early and late RA patients [14], in the prevalence of co-morbidities in women and men [15] and also the gender-specific influence of overweight on disease activity parameters of RA [16] are examples of the association of sociodemographic factors that need to be taken into account in all analyses. The association of gender, education and region of residence was also evaluated in relation to the employment of patients with rheumatic diseases [11].
The decrease in disease activity across all diseases indicates a fundamental improvement in the situation of patients with inflammatory rheumatic diseases. Indirect factors such as the increase in gainful employment [9, 10] and the decline in sick leave and hospitalization confirm a lower burden of illness today. A reduction in lost working days to less than one third is a dramatic change that not only reflects a higher quality of life for those affected, but also significant economic savings. These developments are evident for patients with RA [5], AS [6], PsA and SLE [7, 17].
Today, the disease burden in patients with inflammatory rheumatic diseases is significantly lower
In the 1990s patient-repsorted outcomes that significantly restrict the quality of life of patients, such as pain, fatigue, sleep disorders and psychological stress were reported as early on as the 1990s. An analysis of the ACR/EULAR remission criteria developed in 2011 showed that pain and fatigue were often the main reasons why the new remission criteria were not met, despite fulfilling DAS28 remission [18]. In addition to the recording of disease activity and damage, patient-reported outcomes are now an established and indispensable measure for evaluating the burden of disease and are increasingly being recorded on a disease-specific basis. At this point, the NDB as a disease-overlapping project has increasing limitations, even if the PsA module and sporadically documented disease-specific questionnaires were used in an attempt to address the peculiarities of individual rheumatic diseases.
The NDB shows significant changes in care services. Nevertheless, some forms of therapy are used surprisingly consistently. The first publication already reported that in 1998 56% of patients received MTX as basic therapy [2]. Today, this share is almost identical at 60% [19]. However, MTX is more commonly used today in combination with biologics and biosimilars. Other DMARD therapies have been replaced by more effective substances. In 1998, almost one tenth of rheumatologically treated patients received parenteral gold [2]; today, gold is only used sporadically. The most relevant change is the proportion of patients that are currently treated with bDMARDs, which reaches almost one third in RA [19] and even half of all AS patients [6].
Alongside the successful changes in therapy, the NDB also reveals deficits in the care service. For many years inadequate implementation of the guidelines in everyday life has been observed[20], which in some patients is reflected in a delayed change of therapy, a high supply of glucocorticoids [21] and an inadequate supply of physical and complementary therapies [22] as well as rehabilitation [23].
A constant topic is the supply of glucocorticoids [24, 25]. A high prescription rate of glucocorticoids has been observed over the years, with dosages above 7.5 mg prednisolone equivalent decreasing and only a few patients being treated with high-dose glucocorticoids today [19]. However, almost 50% of long-term RA and polymyalgia rheumatica (PMR) patients remain on glucocorticoid therapy [19, 21]. Today’s recommendations to use glucocorticoids only as briefly as necessary have not yet established themselves among patients that have been ill for many years.
The NDB reveals not only successful changes in treatment but also deficits in the care service
In comparison to data from clinical studies, the NDB illustrates what happens in everyday rheumatological care. The NDB is also an indispensable supplement in comparison to the biologic registry RABBIT, since the NDB also includes patients that have sufficiently controlled disease activity without any or with their first csDMARD therapy. In the estimation of the frequency of prognostically unfavorable factors, the data of the NDB proved to be very helpful, since there is no selection of a prognostically unfavorable patient cohort as in RABBIT [26].
High practice variation in drug treatment was reported as early on as in the first years of the NDB [3]. Despite the development of numerous guidelines, this has not homogenized over the years. Differences among the participating institutions in the supply of bDMARDs and glucocorticoids and also in outcomes that cannot be explained solely by different patient collectives continue to be observed. Why there are still such great differences at this point remains a topic for discussion.
Limitations and strengths of the NDB
Due to the focus on RA, there are barely any specific instruments for other rheumatic diseases, or they are filled out in an insufficient number of cases, e.g. European Consensus Lupus Activity Measurement (ECLAM) or SLEDAI for SLE. With today’s abundance of disease-specific measuring instruments, cross-disease documentation can no longer provide specificity. On the other hand, non-specific but uniform instruments such as the recording of pain, fatigue and function allow a comparison across diseases, which enables the disease burden of other rheumatic diseases to be classified in comparison to RA [8].
Data collection is carried out via and in addition to routine documentation, and the patients receive additional questionnaires. The recording is handled differently in the facilities depending on availability (rheumatologist, study nurse, assistant, tablet/paper, etc.). A complete registration of all outpatients seen in the facility is aimed at, but not required. In contrast to rheumatological registries (e.g. RABBIT) and clinical studies, there is only limited demand for missing values. Therefore, the completeness of the data in the institutions varies according to the resources available. For some values the documentation is almost complete (e.g. for the FFbH), other parameters are insufficiently filled in and cannot be included in evaluations (e.g. radiological status).
A comprehensive picture of the disease status of patients with rheumatic diseases is only possible through supplementary interpretation of the results from various data sources. The analysis of health insurance data by linking data from patient questionnaires in the PROCLAIR project is a helpful supplement to the NDB, especially in the area of health care services [27]. Comparison with data from PROCLAIR enables a classification at population level. There are many comparable results, especially with regard to patient-reported outcomes of pain and functional status, which confirm the quality of both sources [27]. On the other hand, there are large deviations, e.g. in the degree of DMARD care, which is much higher for rheumatologists in the NDB than in the claims data [28] or in the prevalence of co-morbidity diagnoses [29], which are significantly more frequent in the claims data than in the NDB. Since it is not possible to rule out insufficient reporting in the NDB as well as over-reporting in insurance data, the authors estimate the information provided by rheumatologists on concomitant diseases in the NDB to be somewhat too low and the claims diagnoses in the insurance data to be somewhat too high.
Conclusion
The 25 years of the NDB have provided a comprehensive picture of changes in the care of patients with inflammatory rheumatic diseases. The data are a valuable complement to clinical studies and analyses from observational studies, since they are collected under daily practice conditions. Whether the NDB is still future-proof under the increasing number of specific disease registers also depends on whether the collection of patient data can be standardized in the future and whether it will succeed in a modern digital format.
References
Zink A, Listing J, Klindworth C et al (2001) The national database of the German Collaborative Arthritis Centres: I. Structure, aims, and patients. Ann Rheum Dis 60:199–206
Zink A, Listing J, Niewerth M et al (2001) The national database of the German Collaborative Arthritis Centres: II. Treatment of patients with rheumatoid arthritis. Ann Rheum Dis 60:207–213
Zink A, Listing J, Ziemer S et al (2001) Practice variation in the treatment of rheumatoid arthritis among German rheumatologists. J Rheumatol 28:2201–2208
Zink A, Huscher D, Schneider M (2006) Die Kerndokumentation der Rheumazentren – Bilanz nach 12 Jahren. Z Rheumatol 65(144):146–151
Ziegler S, Huscher D, Karberg K et al (2010) Trends in treatment and outcomes of rheumatoid arthritis in Germany 1997–2007: Results from the National Database of the German Collaborative Arthritis Centres. Ann Rheum Dis 69:1803–1808
Huscher D, Thiele K, Rudwaleit M et al (2015) Trends in treatment and outcomes of ankylosing spondylitis in outpatient rheumatological care in Germany between 2000 and 2012. RMD Open 1:e33
Albrecht K, Huscher D, Richter J et al (2014) Changes in referral, treatment and outcomes in patients with systemic lupus erythematosus in Germany in the 1990s and the 2000s. Lupus Sci Med 1:e59
Zink A, Thiele K, Huscher D et al (2006) Healthcare and burden of disease in psoriatic arthritis. A comparison with rheumatoid arthritis and ankylosing spondylitis. J Rheumatol 33:86–90
Mau W, Listing J, Huscher D et al (2005) Employment across chronic inflammatory rheumatic diseases and comparison with the general population. J Rheumatol 32:721–728
Mau W, Thiele K, Lamprecht J (2014) Trends der Erwerbstätigkeit von Rheumakranken. Ergebnisse aus Sozialversicherungsdaten und Kerndokumentation der Rheumazentren in Deutschland. Z Rheumatol 73:11–19
Mau W, Beyer W, Ehlebracht-König I et al (2008) Krankheitslast. Erste Routineberichterstattung zu sozialmedizinischen Folgen entzündlich-rheumatischer Erkrankungen in Deutschland. Z Rheumatol 67:157–164
Huscher D, Merkesdal S, Thiele K et al (2006) Cost of illness in rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis and systemic lupus erythematosus in Germany. Ann Rheum Dis 65:1175–1183
Huscher D, Mittendorf T, von Hinüber U et al (2015) Evolution of cost structures in rheumatoid arthritis over the past decade. Ann Rheum Dis 74:738–745
Huscher D, Sengler C, Gromnica-Ihle E et al (2013) Clinical presentation, burden of disease and treatment in young-onset and late-onset rheumatoid arthritis: A matched-pairs analysis taking age and disease duration into account. Clin Exp Rheumatol 31:256–262
Albrecht K (2014) Gender-spezifische Unterschiede der Komorbidität bei rheumatoider Arthritis. Z Rheumatol 73:607–614
Albrecht K, Richter A, Callhoff J et al (2016) Body mass index distribution in rheumatoid arthritis: A collaborative analysis from three large German rheumatoid arthritis databases. Arthritis Res Ther 18:149
Zink A, Fischer-Betz R, Thiele K et al (2004) Health care and burden of illness in systemic lupus erythematosus compared to rheumatoid arthritis: Results from the National Database of the German Collaborative Arthritis Centres. Lupus 13:529–536
Thiele K, Huscher D, Bischoff S et al (2013) Performance of the 2011 ACR/EULAR preliminary remission criteria compared with DAS28 remission in unselected patients with rheumatoid arthritis. Ann Rheum Dis 72:1194–1199
Albrecht K, Huscher D, Eidner T et al (2017) Versorgung der rheumatoiden Arthritis 2014. Aktuelle Daten aus der Kerndokumentation. Z Rheumatol 76:50–57
Zink A, Huscher D, Schneider M (2010) Wie leitliniengerecht ist die rheumatologische Versorgung? Anspruch und Wirklichkeit. Z Rheumatol 69:318–326
Albrecht K, Huscher D, Buttgereit F et al (2018) Long-term glucocorticoid treatment in patients with polymyalgia rheumatica, giant cell arteritis, or both diseases: Results from a national rheumatology database. Rheumatol Int 38:569–577
Albrecht K, Huscher D (2017) Verordnen wir ausreichend Physikalische Medizin? Aktuelle Daten aus der Kerndokumentation der Arbeitsgemeinschaft Regionaler Kooperativer Rheumazentren. Akt Rheumatol 42:118–121
Mau W, Beyer W, Ehlebracht-König I et al (2014) Leistungstrends der rehabilitativen rheumatologischen Versorgung in Deutschland. Z Rheumatol 73:139–148
Thiele K, Buttgereit F, Huscher D et al (2005) Current use of glucocorticoids in patients with rheumatoid arthritis in Germany. Arthritis Rheum 53:740–747
Thiele K, Buttgereit F, Huscher D et al (2005) Verordnung von Glucocorticoiden bei rheumatologisch betreuten Patienten mit rheumatoider Arthritis in Deutschland. Z Rheumatol 64:149–154
Albrecht K, Richter A, Meissner Y et al (2017) Wie häufig sind prognostisch ungünstige Faktoren bei Patienten mit rheumatoider Arthritis? Eine Abschätzung anhand von 3 epidemiologischen Kohorten. Z Rheumatol 76:434–442
Albrecht K, Zink A (2018) Versorgungssituation der rheumatoiden Arthritis in Deutschland. Akt Rheumatol 43:369–374
Albrecht K, Luque Ramos A, Callhoff J et al (2018) Ambulante Versorgung und Krankheitslast der rheumatoiden Arthritis. Eine Analyse von Abrechnungsdaten und einer Versichertenbefragung. Z Rheumatol 77:102–112
Luque Ramos A, Redeker I, Hoffmann F et al (2019) Comorbidities in patients with rheumatoid arthritis and their association with patient-reported outcomes: Results of claims data linked to questionnaire survey. J Rheumatol. https://doi.org/10.3899/jrheum.180668
Acknowledgements
We would like to thank all patients who agreed to make their data available to the German national database and who made extensive data evaluation possible by carefully documenting the patient forms. Our thanks also go to all participating rheumatologists and employees in the participating institutions, on behalf of all collaborators: Rieke Alten (Berlin), Martin Aringer (Dresden), Tobias Alexander (Berlin), Frank Behrens (Frankfurt/Main), Thorsten Eidner (Jena), Kathrin Fischer (Greifswald), Jörg Henes (Tübingen), Ulrich von Hinüber and Winfried Demary (Hildesheim), Guido Höse (Stadthagen), Kirsten Karberg (Berlin), Stefan Kleinert, Florian Schuch and Jörg Wendler (Erlangen), Ina Kötter (Hamburg), Andreas Krause (Berlin), Wolfgang Ochs (Bayreuth), Jutta Richter and Matthias Schneider (Düsseldorf), Susanna Späthling-Mestekemper (Munich), Siegfried Wassenberg and Ralf Weier (Ratingen). We thank Katja Thiele, Sascha Bischoff and Gregor Förster for their valuable support in data management.
Funding
The NDB was funded from 1993 to 1999 by the German Federal Ministry of Health and from 1999 to 2007 by the German Federal Ministry of Education and Science within the Competence Network Rheumatism (01GI0344/3). Since 2007 it has been supported by unconditional grants from the German Collaborative Arthritis Centers and by a consortium of 11 pharmaceutical companies in the German Academy for Continuing Medical Education in Rheumatology (AbbVie, Actelion, BMS, GSK, Medac, MSD, Pfizer, Roche, Sanofi-Aventis, UCB). The principal investigators have full academic freedom.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
K. Albrecht, J. Callhoff and A. Zink declare that they have no competing interests.
All the investigations on humans described were carried out with the consent of the competent ethics committee, in accordance with national law and in accordance with the Helsinki Declaration of 1975 (as amended). A declaration of consent is available from all patients involved.
Additional information
Redaktion
A. Zink, Berlin
R.E. Schmidt, Hannover
E. Edelmann, Bad Aibling
Rights and permissions
Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Albrecht, K., Callhoff, J. & Zink, A. Long-term trends in rheumatology care. Z Rheumatol 78 (Suppl 2), 65–72 (2019). https://doi.org/10.1007/s00393-019-0680-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00393-019-0680-1