Abstract
Objectives
Prior studies relating body mass index (BMI) to brain volumes suggest an overall inverse association. However, BMI might not be an ideal marker, as it disregards different fat compartments, which carry different metabolic risks. Therefore, we analyzed MR-based fat depots and their association with gray matter (GM) volumes of brain structures, which show volumetric changes in neurodegenerative diseases.
Methods
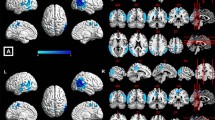
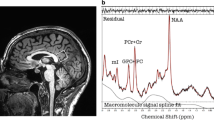
Warp-based automated brain segmentation of 3D FLAIR sequences was obtained in a population-based study cohort. Associations of temporal lobe, cingulate gyrus, and hippocampus GM volume with BMI and MR-based quantification of visceral adipose tissue (VAT), as well as hepatic and pancreatic proton density fat fraction (PDFFhepatic and PDFFpanc, respectively), were assessed by linear regression.
Results
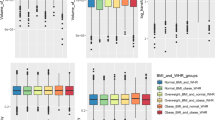
In a sample of 152 women (age 56.2 ± 9.0 years) and 199 men (age 56.1 ± 9.1 years), we observed a significant inverse association of PDFFhepatic and cingulate gyrus volume (p < 0.05) as well as of PDFFhepatic and hippocampus volume (p < 0.05), when adjusting for age and sex. This inverse association was further enhanced for cingulate gyrus volume after additionally adjusting for hypertension, smoking, BMI, LDL, and total cholesterol (p < 0.01) and also alcohol (p < 0.01). No significant association was observed between PDFFhepatic and temporal lobe and between temporal lobe, cingulate gyrus, or hippocampus volume and BMI, VAT, and PDFFpanc.
Conclusions
We observed a significant inverse, independent association of cingulate gyrus and hippocampus GM volume with hepatic fat, but not with other obesity measures. Increased hepatic fat could therefore serve as a marker of high-risk fat distribution.
Key Points
• Obesity is associated with neurodegenerative processes.
• In a population-based study cohort, hepatic fat was superior to BMI and visceral and pancreatic fat as a risk biomarker for decreased brain volume of cingulate gyrus and hippocampus.
• Increased hepatic fat could serve as a marker of high-risk fat distribution.


Similar content being viewed by others
Abbreviations
- AFNI:
-
Analyses of functional images
- BMI:
-
Body mass index
- FAST:
-
FMRIB’s automated segmentation tool
- FLAIR:
-
Fluid attenuation inversion recovery
- FLIR:
-
FMRIB’s linear registration tool
- FMRIB:
-
Functional magnetic resonance imaging of the brain
- FNIRT:
-
FMRIB’s non-linear image registration tool
- GM:
-
Gray matter
- KORA:
-
Cooperative Health Research in the Region of Augsburg
- NAFLD:
-
Nonalcoholic fatty liver disease
- PDFF:
-
Proton density fat fraction
- TBV:
-
Total brain volume
- VIBE:
-
Volume interpolated body examination
- VAT:
-
Visceral adipose tissue
References
Prickett C, Brennan L, Stolwyk R (2015) Examining the relationship between obesity and cognitive function: a systematic literature review. Obes Res Clin Pract 9:93–113
Cornier MA, Marshall JA, Hill JO, Maahs DM, Eckel RH (2011) Prevention of overweight/obesity as a strategy to optimize cardiovascular health. Circulation 124:840–850
Elias MF, Elias PK, Sullivan LM, Wolf PA, D'Agostino RB (2003) Lower cognitive function in the presence of obesity and hypertension: the Framingham heart study. Int J Obes Relat Metab Disord 27:260–268
Gunstad J, Paul RH, Cohen RA, Tate DF, Gordon E (2006) Obesity is associated with memory deficits in young and middle-aged adults. Eat Weight Disord 11:e15–e19
Gustafson D, Lissner L, Bengtsson C, Bjorkelund C, Skoog I (2004) A 24-year follow-up of body mass index and cerebral atrophy. Neurology 63:1876–1881
Bobb JF, Schwartz BS, Davatzikos C, Caffo B (2014) Cross-sectional and longitudinal association of body mass index and brain volume. Hum Brain Mapp 35:75–88
Dhikav V, Anand K (2011) Potential predictors of hippocampal atrophy in Alzheimer’s disease. Drugs Aging 28:1–11
Squire LR, Zola-Morgan S (1991) The medial temporal lobe memory system. Science 253:1380–1386
Driscoll I, Beydoun MA, An Y et al (2012) Midlife obesity and trajectories of brain volume changes in older adults. Hum Brain Mapp 33:2204–2210
Raji CA, Ho AJ, Parikshak NN et al (2010) Brain structure and obesity. Hum Brain Mapp 31:353–364
Weible AP (2013) Remembering to attend: the anterior cingulate cortex and remote memory. Behav Brain Res 245:63–75
Hampson M, Driesen NR, Skudlarski P, Gore JC, Constable RT (2006) Brain connectivity related to working memory performance. J Neurosci 26:13338–13343
Emmerzaal TL, Kiliaan AJ, Gustafson DR (2015) 2003–2013: a decade of body mass index, Alzheimer’s disease, and dementia. J Alzheimers Dis 43:739–755
Pedditizi E, Peters R, Beckett N (2016) The risk of overweight/obesity in mid-life and late life for the development of dementia: a systematic review and meta-analysis of longitudinal studies. Age Ageing 45:14–21
Franzosi MG (2006) Should we continue to use BMI as a cardiovascular risk factor? Lancet 368:624–625
Despres JP (2006) Is visceral obesity the cause of the metabolic syndrome. Ann Med 38:52–63
Smits MM, van Geenen EJ (2011) The clinical significance of pancreatic steatosis. Nat Rev Gastroenterol Hepatol 8:169–177
Yang KC, Hung HF, Lu CW, Chang HH, Lee LT, Huang KC (2016) Association of non-alcoholic fatty liver disease with metabolic syndrome independently of central obesity and insulin resistance. Sci Rep 6:27034
VanWagner LB, Terry JG, Chow LS et al (2017) Nonalcoholic fatty liver disease and measures of early brain health in middle-aged adults: the CARDIA study. Obesity (Silver Spring) 25:642–651
Whitmer RA, Gustafson DR, Barrett-Connor E, Haan MN, Gunderson EP, Yaffe K (2008) Central obesity and increased risk of dementia more than three decades later. Neurology 71:1057–1064
Holle R, Happich M, Lowel H, WichmannHE (2005) KORA--a research platform for population based health research. Gesundheitswesen 67(Suppl 1):S19–S25
Bamberg F, Hetterich H, Rospleszcz S et al (2017) Subclinical disease burden as assessed by whole-body MRI in subjects with prediabetes, subjects with diabetes, and normal control subjects from the general population: the KORA-MRI study. Diabetes 66:158–169
World Health Organization (2006) Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia. World Health Organization, Geneva
Ruf E, Baumert J, Meisinger C, Doring A, Ladwig KH (2014) Are psychosocial stressors associated with the relationship of alcohol consumption and all-cause mortality? BMC Public Health 14:312
Hetterich H, Bayerl C, Peters A et al (2016) Feasibility of a three-step magnetic resonance imaging approach for the assessment of hepatic steatosis in an asymptomatic study population. Eur Radiol 26:1895–1904
Smith SM (2002) Fast robust automated brain extraction. Hum Brain Mapp 17:143–155
Tzourio-Mazoyer N, Landeau B, Papathanassiou D et al (2002) Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15:273–289
Jenkinson M, Bannister P, Brady M, Smith S (2002) Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17:825–841
Zhang C, Cahill ND, Arbabshirani MR, White T, Baum SA, Michael AM (2016) Sex and age effects of functional connectivity in early adulthood. Brain Connect 6:700–713
Beller E, Keeser D, Wehn A et al (2018) T1-MPRAGE and T2-FLAIR segmentation of cortical and subcortical brain regions-an MRI evaluation study. Neuroradiology. https://doi.org/10.1007/s00234-018-2121-2
Wurslin C, Machann J, Rempp H, Claussen C, Yang B, Schick F (2010) Topography mapping of whole body adipose tissue using a fully automated and standardized procedure. J Magn Reson Imaging 31:430–439
Lorbeer R, Bayerl C, Auweter S et al (2017) Association between MRI-derived hepatic fat fraction and blood pressure in participants without history of cardiovascular disease. J Hypertens 35:737–744
Heber SD, Hetterich H, Lorbeer R et al (2017) Pancreatic fat content by magnetic resonance imaging in subjects with prediabetes, diabetes, and controls from a general population without cardiovascular disease. PLoS One 12:e0177154
Dell'Oglio E, Ceccarelli A, Glanz BI et al (2015) Quantification of global cerebral atrophy in multiple sclerosis from 3T MRI using SPM: the role of misclassification errors. J Neuroimaging 25:191–199
Hansen TI, Brezova V, Eikenes L, Haberg A, Vangberg TR (2015) How does the accuracy of intracranial volume measurements affect normalized brain volumes? Sample size estimates based on 966 subjects from the HUNT MRI cohort. AJNR Am J Neuroradiol 36:1450–1456
Pintzka CW, Hansen TI, Evensmoen HR, Haberg AK (2015) Marked effects of intracranial volume correction methods on sex differences in neuroanatomical structures: a HUNT MRI study. Front Neurosci 9:238
Gunstad J, Paul RH, Cohen RA et al (2008) Relationship between body mass index and brain volume in healthy adults. Int J Neurosci 118:1582–1593
Pannacciulli N, Del Parigi A, Chen K, Le DS, Reiman EM, Tataranni PA (2006) Brain abnormalities in human obesity: a voxel-based morphometric study. Neuroimage 31:1419–1425
Fox CS, Massaro JM, Hoffmann U et al (2007) Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation 116:39–48
Cossrow N, Falkner B (2004) Race/ethnic issues in obesity and obesity-related comorbidities. J Clin Endocrinol Metab 89:2590–2594
Barlett HL, Puhl SM, Hodgson JL, Buskirk ER (1991) Fat-free mass in relation to stature: ratios of fat-free mass to height in children, adults, and elderly subjects. Am J Clin Nutr 53:1112–1116
Francque SM, van der Graaff D, Kwanten WJ (2016) Non-alcoholic fatty liver disease and cardiovascular risk: pathophysiological mechanisms and implications. J Hepatol 65:425–443
Wu S, Wu F, Ding Y, Hou J, Bi J, Zhang Z (2016) Association of non-alcoholic fatty liver disease with major adverse cardiovascular events: a systematic review and meta-analysis. Sci Rep 6:33386
Long MT, Wang N, Larson MG et al (2015) Nonalcoholic fatty liver disease and vascular function: cross-sectional analysis in the Framingham heart study. Arterioscler Thromb Vasc Biol 35:1284–1291
Gorelick PB, Scuteri A, Black SE et al (2011) Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American heart association/American stroke association. Stroke 42:2672–2713
Debette S, Seshadri S, Beiser A et al (2011) Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology 77:461–468
Taipale T, Seppala I, Raitoharju E et al (2018) Fatty liver is associated with blood pathways of inflammatory response, immune system activation and prothrombotic state in Young Finns Study. Sci Rep 8:10358
Ghareeb DA, Hafez HS, Hussien HM, Kabapy NF (2011) Non-alcoholic fatty liver induces insulin resistance and metabolic disorders with development of brain damage and dysfunction. Metab Brain Dis 26:253–267
Sapmaz F, Uzman M, Basyigit S et al (2016) Steatosis grade is the Most important risk factor for development of endothelial dysfunction in NAFLD. Medicine (Baltimore) 95:e3280
Stefan N, Haring HU (2013) The role of hepatokines in metabolism. Nat Rev Endocrinol 9:144–152
Weinstein G, Zelber-Sagi S, Preis SR et al (2018) Association of nonalcoholic fatty liver disease with lower brain volume in healthy middle-aged adults in the Framingham Study. JAMA Neurol 75:97–104
Sala M, van der Grond J, de Mutsert R et al (2016) Liver fat assessed with CT relates to MRI markers of incipient brain injury in middle-aged to elderly overweight persons. AJR Am J Roentgenol 206:1087–1092
Blaak E (2001) Gender differences in fat metabolism. Curr Opin Clin Nutr Metab Care 4:499–502
Karastergiou K, Smith SR, Greenberg AS, Fried SK (2012) Sex differences in human adipose tissues - the biology of pear shape. Biol Sex Differ 3:13
Bos D, Leening MJG (2018) Leveraging the coronary calcium scan beyond the coronary calcium score. Eur Radiol 28:3082–3087
Graffy PM, Pickhardt PJ (2016) Quantification of hepatic and visceral fat by CT and MR imaging: relevance to the obesity epidemic, metabolic syndrome and NAFLD. Br J Radiol 89:20151024
Ajmera V, Park CC, Caussy C et al (2018) Magnetic resonance imaging proton density fat fraction associates with progression of fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology 155:307–310 e302
Idilman IS, Aniktar H, Idilman R et al (2013) Hepatic steatosis: quantification by proton density fat fraction with MR imaging versus liver biopsy. Radiology 267:767–775
Lindig T, Kotikalapudi R, Schweikardt D et al (2017) Evaluation of multimodal segmentation based on 3D T1-, T2- and FLAIR-weighted images - the difficulty of choosing. Neuroimage. https://doi.org/10.1016/j.neuroimage.2017.02.016
Funding
This research was funded in-part by the German Research Foundation (DFG, Bonn, Germany; grant-number 245222810). The KORA study was initiated and financed by the Helmholtz Zentrum München—German Research Center for Environmental Health, which is funded by the German Federal Ministry of Education and Research (BMBF) and by the State of Bavaria. The KORA-MRI sub-study was supported by an unrestricted research grant from Siemens Healthcare.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Sophia Stoecklein.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• Retrospective
• Diagnostic or prognostic study
• Performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Beller, E., Lorbeer, R., Keeser, D. et al. Hepatic fat is superior to BMI, visceral and pancreatic fat as a potential risk biomarker for neurodegenerative disease. Eur Radiol 29, 6662–6670 (2019). https://doi.org/10.1007/s00330-019-06276-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-019-06276-8