Abstract
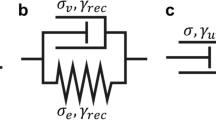
Cells and tissues exhibit sustained oscillatory deformations during remodelling, migration or embryogenesis. Although it has been shown that these oscillations correlate with intracellular biochemical signalling, the role of these oscillations is as yet unclear, and whether they may trigger drastic cell reorganisation events or instabilities remains unknown. Here, we present a rheological model that incorporates elastic, viscous and frictional components, and that is able to generate oscillatory response through a delay adaptive process of the rest-length. We analyse its stability as a function of the model parameters and deduce analytical bounds of the stable domain. While increasing values of the delay and remodelling rate render the model unstable, we also show that increasing friction with the substrate destabilises the oscillatory response. This fact was unexpected and still needs to be verified experimentally. Furthermore, we numerically verify that the extension of the model with non-linear deformation measures is able to generate sustained oscillations converging towards a limit cycle. We interpret this sustained regime in terms of non-linear time varying stiffness parameters that alternate between stable and unstable regions of the linear model. We also note that this limit cycle is not present in the linear model. We study the phase diagram and the bifurcations of the non-linear model, based on our conclusions on the linear one. Such dynamic analysis of the delay visco-elastic model in the presence of friction is absent in the literature for both linear and non-linear rheologies. Our work also shows how increasing values of some parameters such as delay and friction decrease its stability, while other parameters such as stiffness stabilise the oscillatory response.










Similar content being viewed by others
References
Alarcón T, Getto P, Nakata Y (2014) Stability analysis of a renewal equation for cell population dynamics with quiescence. SIAM J Appl Math 74(4):1266–1297
Asl FM, Ulsoy AG (2003) Analysis of a system of linear delay differential equations. J Dyn Sys Meas Contr 125:215–223
Borja C, Moral E, Muñoz JJ (2021) Viscoelasticity and Collective Cell Migration: An interdisciplinary perspective across levels of organization, chap. 5: Effects of time delays and viscoelastic parameters in oscillatory response cell monolayers. Elsevier. https://doi.org/10.1016/C2019-0-01440-9
Cavanaugh K, Staddon M, Munro E, Banerjee S, Gardel M (2020) RhoA mediates epithelial cell shape changes via mechanosensitive endocytosis. Dev Cell 52(2):152–166
Christodoulou N, Skourides P (2015) Cell-autonomous Ca2+ flashes elicit pulsed contractions of an apical actin network to drive apical constriction during neural tube closure 13, 2189–2202
Clément R, Collinet C, Dehapiot B, Lecuit T, Lenne P (2017) Viscoelastic dissipation stabilizes cell shape changes during tissue morphogenesis. Current Biol 27(20):3132–3142
Corless R, Gonnet G, Hare D, Jeffrey D, Knuth D (1996) On the Lambert W function. Adv Comp Math 5:329–359
Dierkes K, Sumi A, Solon J, Salbreux G (2014) Spontaneous oscillations of elastic contractile materials with turnover. Phys Rev Lett 113(148):102
Doubrovinski K, Swan M, Polyakov O, Wieschaus E (2017) Measurement of cortical elasticity in drosophila melanogaster embryos using ferrofluids. Proc Natl Acad Sci USA 114(5):1051–1056
Engelborghs K, Luzyanina T, Roose D (2002) Numerical bifurcation analysis of delay differential equations using DDE-BIFTOOL. ACM Trans Math Soft 28(1):1–21
Erlich A, Jones G, Tisseur F, Moulton D, Goriely A (2020) The role of topology and mechanics in uniaxially growing cell networks. Proc R Soc A 4762233), ID: 20190523
Erneux T (2009) Applied delay differential equations, surveys and tutorials in the applied mathematical sciences, vol 3. Springer, New York
Getto P, Gyllenberg M, Nakata Y, Scarabel F (2019) Stability analysis of a state-dependent delay differential equation for cell maturation: analytical and numerical methods. J Math Biol 79:281–328
Goriely A (2017) Morphoelasticity: the mathematics and mechanics of biological growth. Springer interdisciplinary and applied mathematics. Springer, Berlin
Guckenheimer J, Holmes P (1983) Nonlinear oscillations, dynamicl systems, and bifurcations of vector fields, Applied Mathematical Sciences, vol 42. Springer, Berlin
Gyllenberg M, Heijmans HJAM (1987) An abstract delay-differential equation modelling size dependent cell growth and division. SIAM J Math Anal 18(1):74–88
Insperger T, Stépán G (2002) Semi-discretization method for delayed systems. Int J Num Meth Eng 55(5):503–518
Kaouri K, Maini PK, Skourides PA, Christodoulou N, Chapman SJ (2019) A simple mechanochemical model for calcium signalling in embryonic epithelial cells. J Math Biol 78:2059–2092
Karkali K, Tiwari P, Singh A, Tlili S, Jorba I, Navajas D, Muñoz JJ, Saunders T, Martín-Blanco E (2021) Condensation of the drosophila nerve cord is oscillatory and depends on coordinated mechanical interactions. bioRxiv. https://doi.org/10.1101/2021.02.24.432750
Khalilgharibi N, Fouchard J, Asadipour N, Barrientos R, Duda M, Bonfanti A, Yonis A, Harris A, Mosaffa P, Fujita Y, Kabla A, Mao Y, Baum B, Muñoz JJ, Miodownik M, Charras G (2019) Stress relaxation in epithelial monolayers is controlled by the actomyosin cortex. Nat Phys 15:839–847
Lapytsko A, Schaber J (2016) The role of time delay in adaptive cellular negative feedback systems. J Theor Biol 308:64–73
Martin AC, Kaschube M, Wieschaus EF (2009) Pulsed contractions of an actin-myosin network drive apical constriction. Nature 457:495–499
Mosaffa P, Tetley RJ, Rodríguez-Ferran A, Mao Y, Muñoz JJ (2020) Junctional and cytoplasmic contributions in wound healing. J R Soc Interface 17(20200):264
Muñoz JJ, Albo S (2013) Physiology-based model of cell viscoelasticity. Phys Rev E 88(1):012708
Muñoz JJ, Conte V, Miodownik M (2010) Stress dependent morphogenesis: continuum mechanics and truss systems. Biomech Model Mechanobiol 9(4):451–467
Muñoz JJ, Dingle M, Wenzel M (2018) Mechanical oscillations in biological tissues as a result of delayed rest-length changes. Phys Rev E 98(1):052409
Petrolli V, Goff M, Tadrous M, Martens K, Allier C, Mandula O, Hervé L, Henkes S, Sknepnek R, Boudou T, Cappello G, Balland M (2019) Confinement-induced transition between wave-like collective cell migration modes. Phys Rev Lett 122(16):168101. https://doi.org/10.1101/495747
Petrungaro G, Morelli L, Uriu K (2019) Information flow in the presence of cell mixing and signalling delays during embryonic development. Sem Cell Dev Biol 93:23–35
Peyret G, Mueller R, d’Alessandro J, Begnaud S, Marcq P, Mège R, Yeomans J, Doostmohammadi A, Ladoux B (2019) Sustained oscillations of epithelial cell sheets. Bioph J 117(3):454–478
Roldán L, Muñoz JJ, Sáez P (2019) Computational modeling of epithelial wound healing: Short and long term chemo–mechanical mechanisms. Comp Meth Appl Mech Eng 350:25–56
Shinozaki H, Mori T (2006) Robust stability analysis of linear time-delay systems by Lambert W function: some extreme point results. Automatica 42(1):1791–1799
Smith H (2011) An introduction to delay differential equations with applications to the life sciences. Texts in applied mathematics. Springer, New York
Solon J, Kaya-Copur A, Brunner D (2009) Pulsed forces timed by a ratchet-like mechanism drive directed tissue movement during dorsal closure. Cell 58(137):1331–1342
Stépán G (1989) Retarded dynamical systems: stability and characteristic functions, Pitman Res. Notes Math., vol. 210. Longman Scientific & Technical, Essex, UK
Suzuki M, Sato M, Koyama H, Hara Y, Hayashi K, Yasue N, Imamura H, Fujimori T, Nagai T, Campbell R, Ueno N (2017) Distinct intracellular Ca2+ dynamics regulate apical constriction and differentially contribute to neural tube closure. Development 144:1307–1316
Sykora H, Bachrathy D, Stépán G (2019) Stochastic semi-discretization for linear stochastic delay differential equations. Int J Num Meth Eng 119(9):879–898
Taber LA (2008) Theoretical study of beloussov’s hyper-restoration hypothesis for mechanical regulation of morphogenesis. Biomech Model Mechanobiol 7(8):427–441
Tao H, Zhu M, Lau K, Whitley OW, Samani M, Xiao X, Chen X, Hahn N, Liu W, Valencia M, Wu M, Wang X, Fenelon K, Pasiliao C, Hu D, Wu J, Spring S, Ferguson J, Karuna E, Henkelman R, Dunn A, Huang H, Ho H, Atit R, Goyal S, Sun Y, Hopyan S (2019) Oscillatory cortical forces promote three dimensional cell intercalations that shape the murine mandibular arch. Nat Commun 10(1703):1–18
Wyatt TJ, Fouchard J, Lisica A, Khalilgharibi N, Baum B, Recho P, Kabla A, Charras G (2020) Actomyosin controls planarity and folding of epithelia in response to compression. Num Math 19:109–117
Yoshioka-Kobayashi K, Matsumiya M, Niino Y, Isomura A, Kori H, Miyawaki A, Kageyama R (2020) Coupling delay controls synchronized oscillation in the segmentation clock. Nature 580(7801):119–123
Zulueta-Coarasa T, Fernandez-Gonzalez R (2018) Dynamic force patterns promote collective cell movements during embryonic wound repair. Nat Phys 14:750–758
Acknowledgements
JJM and MD have been financially supported by the Spanish Ministry of Science, Innovation and Universities (MICINN) with grant DPI2016-74929-R and by the local government Generalitat de Catalunya with grant 2017 SGR 1278.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dawi, M.A., Muñoz, J.J. Stability bounds of a delay visco-elastic rheological model with substrate friction. J. Math. Biol. 83, 71 (2021). https://doi.org/10.1007/s00285-021-01699-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00285-021-01699-8