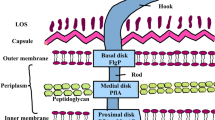
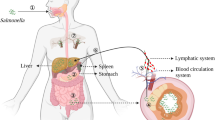
Abstract
Amoebic bacterial interactions are the most ancient form of host pathogen interactions. Here, we investigate the fate of Salmonella typhimurium and Acanthamoeba castellanii T4 genotype upon mutual interactions in a nutrition free environment. The role of type 1 fimbriae and motility of S. typhimurium during interactions with A. castellanii has also been investigated. Deletion of genes encoding the type 1 fimbriae subunit FimA, type 1 fimbriae tip protein FimH, chemotaxis regulatory proteins CheA and CheY and major flagella subunits FliC and FljB was performed through homologous recombination. In vitro association, invasion and survival assays of S. typhimurium wild-type and mutant strains were performed upon co-incubation of bacteria with A. castellanii trophozoites in a nutrition free environment. The deletion gene encoding type 1 fimbriae subunit FimA reduced, whereas the deletion of genes encoding flagella subunits FliC and FljB of flagella enhanced the association capability of S. typhimurium with A. castellanii. Invasion of A. castellanii by Salmonella was significantly reduced upon the loss of type 1 fimbriae subunit FimA and type 1 fimbriae tip protein FimH. Co-incubation of S. typhimurium with A. castellanii in phosphate buffered saline medium stimulated the growth of S. typhimurium wild-type and mutant strains. Viable A. castellanii trophozoites count became significantly reduced upon co-incubation with S. typhimurium within 48 h. Type 1 fimbriae play a pivotal role in the adherence of S. typhimurium to the A. castellanii cell surface. Subsequently, this interaction provides S. typhimurium an advantage in growth.





Similar content being viewed by others
References
Thomas V, Herrera-Rimann K, Blanc DS, Greub G (2006) Biodiversity of amoebae and amoeba-resisting bacteria in a hospital water network. Appl Environ Microbiol 72(4):2428–2438. https://doi.org/10.1128/aem.72.4.2428-2438.2006
Cateau E, Delafont V, Hechard Y, Rodier MH (2014) Free-living amoebae: what part do they play in healthcare-associated infections? J Hosp Infect 87(3):131–140. https://doi.org/10.1016/j.jhin.2014.05.001
Scheid P (2014) Relevance of free-living amoebae as hosts for phylogenetically diverse microorganisms. Parasitol Res 113(7):2407–2414. https://doi.org/10.1007/s00436-014-3932-7
Muchesa P, Leifels M, Jurzik L, Hoorzook KB, Barnard TG, Bartie C (2017) Coexistence of free-living amoebae and bacteria in selected South African hospital water distribution systems. Parasitol Res 116(1):155–165. https://doi.org/10.1007/s00436-016-5271-3
Amann R, Springer N, Schonhuber W, Ludwig W, Schmid EN, Muller KD, Michel R (1997) Obligate intracellular bacterial parasites of acanthamoebae related to Chlamydia spp. Appl Environ Microbiol 63(1):115–121
Steinert M, Birkness K, White E, Fields B, Quinn F (1998) Mycobacterium avium bacilli grow saprozoically in coculture with Acanthamoeba polyphaga and survive within cyst walls. Appl Environ Microbiol 64(6):2256–2261
Haas A (2007) The phagosome: compartment with a license to kill. Traffic (Copenhagen, Denmark) 8(4):311–330. https://doi.org/10.1111/j.1600-0854.2006.00531.x
Cosson P, Lima WC (2014) Intracellular killing of bacteria: is Dictyostelium a model macrophage or an alien? Cell Microbiol 16(6):816–823
Steinert M (2011) Pathogen-host interactions in Dictyostelium, Legionella, Mycobacterium and other pathogens. Semin Cell Dev Biol 22(1):70–76
Tosetti N, Croxatto A, Greub G (2014) Amoebae as a tool to isolate new bacterial species, to discover new virulence factors and to study the host-pathogen interactions. Microb Pathog 77:125–130
Gaze WH, Burroughs N, Gallagher MP, Wellington EM (2003) Interactions between Salmonella typhimurium and Acanthamoeba polyphaga, and observation of a new mode of intracellular growth within contractile vacuoles. Microb Ecol 46(3):358–369. https://doi.org/10.1007/s00248-003-1001-3
Tezcan-Merdol D, Ljungstrom M, Winiecka-Krusnell J, Linder E, Engstrand L, Rhen M (2004) Uptake and replication of Salmonella enterica in Acanthamoeba rhysodes. Appl Environ Microbiol 70(6):3706–3714. https://doi.org/10.1128/aem.70.6.3706-3714.2004
Gill MA, Rafique MW, Manan T, Slaeem S, Romling U, Matin A, Ahmad I (2018) The cellulose synthase BcsA plays a role in interactions of Salmonella typhimurium with Acanthamoeba castellanii genotype T4. Parasitol Res 117(7):2283–2289. https://doi.org/10.1007/s00436-018-5917-4
Van der Henst C, Vanhove AS, Drebes Dorr NC, Stutzmann S, Stoudmann C, Clerc S, Scrignari T, Maclachlan C, Knott G, Blokesch M (2018) Molecular insights into Vibrio cholerae's intra-amoebal host-pathogen interactions. Nat Commun 9(1):3460. https://doi.org/10.1038/s41467-018-05976-x
Rude RA, Jackson GJ, Bier JW, Sawyer TK, Risty NG (1984) Survey of fresh vegetables for nematodes, amoebae, and Salmonella. J Assoc Off Anal Chem 67(3):613–615
Jensen VB, Harty JT, Jones BD (1998) Interactions of the invasive pathogens Salmonella typhimurium, Listeria monocytogenes, and Shigella flexneri with M cells and murine Peyer's patches. Infect Immun 66(8):3758–3766
Jones BD, Ghori N, Falkow S (1994) Salmonella typhimurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer's patches. J Exp Med 180(1):15–23
Martinez AJ, Visvesvara GS (1997) Free-living, amphizoic and opportunistic amebas. Brain Pathol (Zurich, Switzerland) 7(1):583–598
Thamprasert K, Khunamornpong S, Morakote N (1993) Acanthamoeba infection of peptic ulcer. Ann Trop Med Parasitol 87(4):403–405
Zaman V, Zaki M, Manzoor M (1999) Acanthamoeba in human faeces from Karachi. Ann Trop Med Parasitol 93(2):189–191
King CH, Shotts EB Jr, Wooley RE, Porter KG (1988) Survival of coliforms and bacterial pathogens within protozoa during chlorination. Appl Environ Microbiol 54(12):3023–3033
Kolenda R, Ugorski M, Grzymajlo K (2019) Everything you always wanted to know about Salmonella type 1 fimbriae, but were afraid to ask. Front Microbiol 10:1017. https://doi.org/10.3389/fmicb.2019.01017
Duguid JP, Campbell I (1969) Antigens of the type-1 fimbriae of salmonellae and other enterobacteria. J Med Microbiol 2(4):535–553. https://doi.org/10.1099/00222615-2-4-535
Nuccio SP, Baumler AJ (2007) Evolution of the chaperone/usher assembly pathway: fimbrial classification goes Greek. Microbiol Mol Biol Rev 71(4):551–575. https://doi.org/10.1128/mmbr.00014-07
Althouse C, Patterson S, Fedorka-Cray P, Isaacson RE (2003) Type 1 fimbriae of Salmonella enterica serovar Typhimurium bind to enterocytes and contribute to colonization of swine in vivo. Infect Immun 71(11):6446–6452
Ewen SW, Naughton PJ, Grant G, Sojka M, Allen-Vercoe E, Bardocz S, Thorns CJ, Pusztai A (1997) Salmonella enterica var Typhimurium and Salmonella enterica var Enteritidis express type 1 fimbriae in the rat in vivo. FEMS Immunol Med Microbiol 18(3):185–192
Swenson DL, Kim KJ, Six EW, Clegg S (1994) The gene fimU affects expression of Salmonella typhimurium type 1 fimbriae and is related to the Escherichia coli tRNA gene argU. Mol Gen Genet 244(2):216–218
Kisiela DI, Kramer JJ, Tchesnokova V, Aprikian P, Yarov-Yarovoy V, Clegg S, Sokurenko EV (2011) Allosteric catch bond properties of the FimH adhesin from Salmonella enterica serovar Typhimurium. J Biol Chem 286(44):38136–38147. https://doi.org/10.1074/jbc.M111.237511
Garate M, Cubillos I, Marchant J, Panjwani N (2005) Biochemical characterization and functional studies of Acanthamoeba mannose-binding protein. Infect Immun 73(9):5775–5781. https://doi.org/10.1128/iai.73.9.5775-5781.2005
Krell T, Lacal J, Munoz-Martinez F, Reyes-Darias JA, Cadirci BH, Garcia-Fontana C, Ramos JL (2011) Diversity at its best: bacterial taxis. Environ Microbiol 13(5):1115–1124. https://doi.org/10.1111/j.1462-2920.2010.02383.x
Park SY, Borbat PP, Gonzalez-Bonet G, Bhatnagar J, Pollard AM, Freed JH, Bilwes AM, Crane BR (2006) Reconstruction of the chemotaxis receptor-kinase assembly. Nat Struct Mol Biol 13(5):400–407. https://doi.org/10.1038/nsmb1085
Erbse AH, Falke JJ (2009) The core signaling proteins of bacterial chemotaxis assemble to form an ultrastable complex. Biochemistry 48(29):6975–6987. https://doi.org/10.1021/bi900641c
Borkovich KA, Kaplan N, Hess JF, Simon MI (1989) Transmembrane signal transduction in bacterial chemotaxis involves ligand-dependent activation of phosphate group transfer. Proc Natl Acad Sci USA 86(4):1208–1212
Garrity LF, Ordal GW (1997) Activation of the CheA kinase by asparagine in Bacillus subtilis chemotaxis. Microbiology (Reading, England) 143(Pt 9):2945–2951. https://doi.org/10.1099/00221287-143-9-2945
Sowa Y, Berry RM (2008) Bacterial flagellar motor. Q Rev Biophys 41(2):103–132. https://doi.org/10.1017/s0033583508004691
Shoaib HM, Muazzam AG, Mir A, Jung S-Y, Matin A (2013) Evaluation of inhibitory potential of some selective methanolic plants extracts on biological characteristics of Acanthamoeba castellanii using human corneal epithelial cells in vitro. Parasitol Res 112(3):1179–1188
Jung SY, Matin A, Kim KS, Khan NA (2007) The capsule plays an important role in Escherichia coli K1 interactions with Acanthamoeba. Int J Parasitol 37(3–4):417–423. https://doi.org/10.1016/j.ijpara.2006.10.012
Datsenko KA, Wanner BL (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA 97(12):6640–6645
Huws SA, Morley RJ, Jones MV, Brown MR, Smith AW (2008) Interactions of some common pathogenic bacteria with Acanthamoeba polyphaga. FEMS Microbiol Lett 282(2):258–265
Snelling WJ, Moore JE, McKenna JP, Lecky DM, Dooley JS (2006) Bacterial-protozoa interactions; an update on the role these phenomena play towards human illness. Microbes Infect 8(2):578–587
Pernthaler J (2005) Predation on prokaryotes in the water column and its ecological implications. Nat Rev Microbiol 3(7):537–546. https://doi.org/10.1038/nrmicro1180
Matz C, Kjelleberg S (2005) Off the hook–how bacteria survive protozoan grazing. Trends Microbiol 13(7):302–307. https://doi.org/10.1016/j.tim.2005.05.009
Douesnard-Malo F, Daigle F (2011) Increased persistence of Salmonella enterica serovar Typhi in the presence of Acanthamoeba castellanii. Appl Environ Microbiol 77(21):7640–7646. https://doi.org/10.1128/aem.00699-11
Simm R, Ahmad I, Rhen M, Le Guyon S, Romling U (2014) Regulation of biofilm formation in Salmonella enterica serovar Typhimurium. Future Microbiol 9(11):1261–1282
Malaviya R, Ross E, Jakschik BA, Abraham SN (1994) Mast cell degranulation induced by type 1 fimbriated Escherichia coli in mice. J Clin Invest 93(4):1645–1653
Ofek I, Mirelman D, Sharon N (1977) Adherence of Escherichia coli to human mucosal cells mediated by mannose receptors. Nature 265(5595):623–625
Sakarya S, Ertem GT, Oncu S, Kocak I, Erol N (2003) Escherichia coli bind to urinary bladder epithelium through nonspecific sialic acid mediated adherence. FEMS Immunol Med Microbiol 39(1):45–50
Gerlach RG, Claudio N, Rohde M, Jackel D, Wagner C, Hensel M (2008) Cooperation of Salmonella pathogenicity islands 1 and 4 is required to breach epithelial barriers. Cell Microbiol 10(11):2364–2376
Stone BJ, Abu Kwaik Y (1998) Expression of multiple pili by Legionella pneumophila: identification and characterization of a type IV pilin gene and its role in adherence to mammalian and protozoan cells. Infect Immun 66(4):1768–1775
Ahmad I, Wigren E, Le Guyon S, Vekkeli S, Blanka A, El Mouali Y, Anwar N, Chuah ML, Lunsdorf H, Frank R, Rhen M, Liang ZX, Lindqvist Y, Romling U (2013) The EAL-like protein STM1697 regulates virulence phenotypes, motility and biofilm formation in Salmonella typhimurium. Mol Microbiol 90(6):1216–1232
Galan JE (2001) Salmonella interactions with host cells: type III secretion at work. Annu Rev Cell Dev Biol 17:53–86. https://doi.org/10.1146/annurev.cellbio.17.1.53
Misselwitz B, Kreibich SK, Rout S, Stecher B, Periaswamy B, Hardt WD (2011) Salmonella enterica serovar Typhimurium binds to HeLa cells via Fim-mediated reversible adhesion and irreversible type three secretion system 1-mediated docking. Infect Immun 79(1):330–341
Olsen JE, Hoegh-Andersen KH, Casadesus J, Thomsen LE (2012) The importance of motility and chemotaxis for extra-animal survival of Salmonella enterica serovar Typhimurium and Dublin. J Appl Microbiol 113(3):560–568. https://doi.org/10.1111/j.1365-2672.2012.05363.x
Acknowledgements
We are grateful to Prof Ute Romling for reading the manuscript and providing tremendous feed back to improve its contents.
Funding
This work was funded by University of Health Sciences to support thesis work of TM, MWR and MHB.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Research Involving Human and Animal Participants
The study does not involve humans or animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mannan, T., Rafique, M.W., Bhatti, M.H. et al. Type 1 Fimbriae and Motility Play a Pivotal Role During Interactions of Salmonella typhimurium with Acanthamoeba castellanii (T4 Genotype). Curr Microbiol 77, 836–845 (2020). https://doi.org/10.1007/s00284-019-01868-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-019-01868-5