Abstract
Purpose
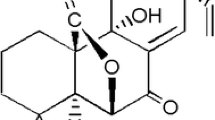
Evasion to new treatments of advanced melanoma is still associated with a poor prognosis. Choosing the best combination of agents that can bypass resistance mechanisms remains a challenge. Sphaeropsidin A (Sph A) is a fungal bioactive secondary metabolite previously shown to force melanoma cells to undergo apoptosis via cell volume dysregulation. This work studied its in vitro combination with cytotoxic chemotherapeutics in a rational manner.
Methods
Four melanoma cell lines harboring different sensitivity levels to pro-apoptotic stimuli were used to build a predictive response surface model allowing the determination of the optimal in vitro combinations of Sph A with two drugs, i.e., cisplatin or temozolomide, owing to a limited set of experimentations.
Results
Testing 12 experimental combinations allowed us to build an accurate predictive model that considers the complexity of the drug interaction and determines the optimal combinations according to the endpoint chosen, i.e., the maximal cytotoxic effects. Therefore, combining 4 µM Sph A with 75 µM cisplatin concomitantly for 72 h improved its cytotoxic effects on melanoma cells in a synergistic manner. An optimal in vitro treatment schedule was also obtained for temozolomide.
Conclusions
The use of a response surface model offers the possibility of reducing the experiments while determining accurately the optimal combinations. We herein highlighted that combining the Na+/K+/2Cl− cotransporter and/or anion exchanger inhibitor Sph A with chemotherapeutic agents could improve the therapeutic benefits of conventional chemotherapies against advanced melanomas, particularly because Sph A exerts cytotoxic effects regardless of the genetic BRAF and NRAS status.




Similar content being viewed by others
Abbreviations
- ADME:
-
Absorption, distribution, metabolism, and excretion
- AUC:
-
Area under the curve
- CDDP:
-
Cisplatin
- Cmax:
-
Maximum (or peak) serum concentration
- CTLA-4:
-
Cytotoxic T lymphocyte antigen-4
- DTIC:
-
Dacarbazine
- EDTA:
-
Ethylenediaminetetraacetic acid
- GI50 :
-
Growth inhibition 50%
- GST:
-
Glutathione-S-transferase
- LC50/90 :
-
Lethal concentration 50/90
- MCDM:
-
Multi-criteria decision-making
- MDR:
-
Multidrug resistance
- MTIC:
-
5-(3-methyltriazen-1-yl)imidazole-4-carboxamide
- MTT:
-
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NCI:
-
National cancer institute
- NKCC1/2:
-
Na+–K+–2Cl− co-transporters ½
- PBS:
-
Phosphate buffered saline
- PD1:
-
Programmed cell death protein 1
- PFS:
-
Progression-free survival
- RVI:
-
Regulatory volume increase
- Sph A:
-
Sphaeropsidin A
- TMZ:
-
Temozolomide
References
Long GV, Weber JS, Infante JR et al (2016) Overall survival and durable responses in patients with BRAF V600-mutant metastatic melanoma receiving dabrafenib combined with trametinib. J Clin Oncol 34(8):871–878. doi:10.1200/JCO.2015.62.9345
Long GV, Stroyakovskiy D, Gogas H et al (2014) Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med 371:1877–1888. doi:10.1056/NEJMoa1406037
Welsh SJ, Rizos H, Scolyer RA, Long GV (2016) Resistance to combination BRAF and MEK inhibition in metastatic melanoma: where to next? Eur J Cancer 62:76–85. doi:10.1016/j.ejca.2016.04.005
Chapman PB, Hauschild A, Robert C et al (2011) Improved survival with vemurafenic in melanoma with BRAF V600E mutation. N Eng J Med 364:2507–2516. doi:10.1056/NEJMoa1103782.Improved
Hauschild A, Grob JJ, Demidov LV et al (2012) Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet 380:358–365. doi:10.1016/S0140-6736(12)60868-X
Larkin J, Chiarion-Sileni V, Gonzalez R et al (2015) Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 373:23–34. doi:10.1056/NEJMoa1504030
Harries M, Malvehy J, Lebbe C et al (2016) Treatment patterns of advanced malignant melanoma (stage III–IV) – a review of current standards in Europe. Eur J Cancer 60:179–189. doi:10.1016/j.ejca.2016.01.011
Grazia G, Penna I, Perotti V et al (2014) Towards combinatorial targeted therapy in melanoma: from pre-clinical evidence to clinical application (review). Int J Oncol 45:929–949. doi:10.3892/ijo.2014.2491
Charles EM, Rehm M (2014) Key regulators of apoptosis execution as biomarker candidates in melanoma. Mol Cell Oncol 1:37–41. doi:10.4161/23723548.2014.964037
Dasari S, Tchounwou PB (2014) Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol 0:364–378. doi:10.1016/j.ejphar.2014.07.025.Cisplatin
Quirt I, Verma S, Petrella T et al (2007) Temozolomide for the treatment of metastatic melanoma: a systematic review. Oncologist 12:1114–1123. doi:10.1634/theoncologist.12-9-1114
Roos WP, Quiros S, Krumm A et al (2014) B-Raf inhibitor vemurafenib in combination with temozolomide and fotemustine in the killing response of malignant melanoma cells. Oncotarget 5:12607–12620. doi:10.18632/oncotarget.2610
Knizhnik AV, Roos WP, Nikolova T et al (2013) Survival and death strategies in glioma cells: autophagy, senescence and apoptosis triggered by a single type of temozolomide-induced DNA damage. PLoS One 8:1–12. doi:10.1371/journal.pone.0055665
Sparapano L, Bruno G, Fierro O, Evidente A (2004) Studies on structure-activity relationship of sphaeropsidins A-F, phytotoxins produced by Sphaeropsis sapinea f. sp. cupressi. Phytochemistry 65:189–198. doi:10.1016/j.phytochem.2003.11.006
Wang XN, Bashyal BP, Wijeratne EMK et al (2011) Smardaesidins A–G, isopimarane and 20 nor-isopimarane diterpenoids from Smardaea sp., a fungal endophyte of the moss Ceratodon purpureus. J Nat Prod 74:2052–2061. doi:10.1021/np2000864
Mathieu V, Chantôme A, Lefranc F et al (2015) Sphaeropsidin A shows promising activity against drug-resistant cancer cells by targeting regulatory volume increase. Cell Mol Life Sci 72:3731–3746. doi:10.1007/s00018-015-1902-6
Poulsen KA, Andersen EC, Hansen CF et al (2010) Deregulation of apoptotic volume decrease and ionic movements in multidrug-resistant tumor cells: role of chloride channels. Am J Physiol Cell Physiol 298:C14–C25. doi:10.1152/ajpcell.00459.2009
Hoffmann EK (2011) Ion channels involved in cell volume regulation: effects on migration, proliferation, and programmed cell death in non adherent EAT cells and adherent ELA cells. Cell Physiol Biochem 28:1061–1078. doi:10.1159/000335843
Algharabli J, Kintner DB, Wang Q et al (2012) Inhibition of Na + Cell Physiol Biochem -K+ -2Cl–cotransporter isoform 1 accelerates temozolomide-mediated apoptosis in glioblastoma cancer cells. Cell Physiol Biochem 30:33–48. doi:10.1038/nmeth.2250.Digestion
Zhu W, Begum G, Pointer K et al (2014) WNK1-OSR1 kinase-mediated phospho-activation of Na+-K+-2Cl- cotransporter facilitates glioma migration. Mol Cancer 13:1–15. doi:10.1186/1476-4598-13-31
Dejaegher B, Vander Heyden Y (2011) Experimental designs and their recent advances in set-up, data interpretation, and analytical applications. J Pharm Biomed Anal 56:141–158. doi:10.1016/j.jpba.2011.04.023
Berger W, Hauptmann E, Elbling L et al (1997) Possible role of the multidrug resistance-associated protein (MRP) in chemoresistance of human melanoma cells. Int J cancer 71:108–115
Birner P, Berghoff AS, Dinhof C et al (2014) MAP kinase activity supported by BRAF (V600E) mutation rather than gene amplification is associated with ETV1 expression in melanoma brain metastases. Arch Dermatol Res 306:873–884. doi:10.1007/s00403-014-1490-6
Evidente A, Sparapano L, Fierro O et al (1997) Sphaeropsidins B and C, phytotoxic pimarane diterpenes from Sphaeropsis sapinea f. sp. cupressi and Diplodia mutila. Phytochemistry 45:705–713. doi:10.1016/S0031-9422(97)00006-X
Heffeter P, Jakupec MA, Körner W et al (2007) Multidrug-resistant cancer cells are preferential targets of the new antineoplastic lanthanum compound KP772 (FFC24). Biochem Pharmacol 73:1873–1886. doi:10.1016/j.bcp.2007.03.002
Mathieu V, Pirker C, Schmidt WM et al (2012) Aggressiveness of human melanoma xenograft models is promoted by aneuploidy-driven gene expression deregulation. Oncotarget 3:399–413 doi:não achei
Mathieu V, Pirker C, Martin de Lassalle E et al (2009) The sodium pump alpha1 sub-unit: A disease progression-related target for metastatic melanoma treatment. J Cell Mol Med 13:3960–3972. doi:10.1111/j.1582-4934.2009.00708.x
Melnikova VO, Bolshakov SV, Walker C, Ananthaswamy HN (2004) Genomic alterations in spontaneous and carcinogen-induced murine melanoma cell lines. Oncogene 23:2347–2356. doi:10.1038/sj.onc.1207405
Naumann SC, Roos WP, Jöst E et al (2009) Temozolomide- and fotemustine-induced apoptosis in human malignant melanoma cells: response related to MGMT, MMR, DSBs, and p53. Br J Cancer 100:322–233. doi:10.1038/sj.bjc.6604856
Li W, Melton D (2012) Cisplatin regulates the MAPK kinase pathway to induce increased expression of DNA repair gene ERCC1 and increase melanoma chemoresistance. Oncogene 31:2412–2422. doi:10.1038/onc.2011.426
Chen Y-P, Hou X-Y, Yang C-S et al (2016) DNA methylation and histone acetylation regulate the expression of MGMT and chemosensitivity to temozolomide in malignant melanoma cell lines. Tumor Biol. doi:10.1007/s13277-016-4994-1
Derringer G, Suich R (1980) Simultaneous optimization of several response variables. J Qual Technol 12:214–219. doi:10.2116/analsci.22.955
Tanami H, Imoto I, Hirasawa A et al (2004) Involvement of overexpressed wild-type BRAF in the growth of malignant melanoma cell lines. Oncogene 23:8796–8804. doi:10.1038/sj.onc.1208152
Clarke R (1997) Issues in experimental design and endpoint analysis in the study of experimental cytotoxic agents in vivo in breast cancer and other models. Breast Cancer Res Treat 46:255–278. doi:10.1023/A:1005938428456
Garg AD, Vandenberk L, Koks C et al (2016) Dendritic cell vaccines based on immunogenic cell death elicit danger signals and T cell-driven rejection of high-grade glioma. Sci Transl Med 8:328ra327. doi:10.1126/scitranslmed.aae0105
Sullivan RJ, Flaherty KT (2013) Resistance to BRAF-targeted therapy in melanoma. Eur J Cancer 49:1297–1304. doi:10.1016/j.ejca.2012.11.019
Dejaegher B, Vander Heyden Y (2009) The use of experimental design in separation science. Acta Chromatogr 21:161–201. doi:10.1556/AChrom.21.2009.2.1
Candioti LV, De Zan MM, Cámara MS, Goicoechea HC (2014) Experimental design and multiple response optimization. Using the desirability function in analytical methods development. Talanta 124:123–138. doi:10.1016/j.talanta.2014.01.034
Witek-Krowiak A, Chojnacka K, Podstawczyk D et al (2014) Application of response surface methodology and artificial neural network methods in modelling and optimization of biosorption process. Bioresour Technol 160:150–160. doi:10.1016/j.biortech.2014.01.021
Ikeda K, Terashima M, Kawamura H et al (1998) Pharmacokinetics of cisplatin in combined cisplatin and 5-fluorouracil therapy: a comparative study of three different schedules of cisplatin administration. Jpn J Clin Oncol 28:168–175. doi:10.1093/jjco/28.3.168
Yao Z, Torres NM, Tao A et al (2015) BRAF mutants evade ERK-dependent feedback by different mechanisms that determine their sensitivity to pharmacologic inhibition. Cancer Cell 28:370–383. doi:10.1016/j.ccell.2015.08.001
Leonard R, Hennessy BT, Blum JL, O’Shaughnessy J (2011) Dose-adjusting capecitabine minimizes adverse effects while maintaining efficacy: A retrospective review of capecitabine for metastatic breast cancer. Clin Breast Cancer 11:349–356. doi:10.1016/j.clbc.2011.06.005
Kakigi A, Nishimura M, Takeda T et al (2009) Expression of aquaporin1, 3, and 4, NKCC1, and NKCC2 in the human endolymphatic sac. Auris Nasus Larynx 36:135–139. doi:10.1016/j.anl.2008.04.012
Orlov SN, Koltsova S V, Kapilevich L V et al (2015) NKCC1 and NKCC2: the pathogenetic role of cation-chloride cotransporters in hypertension. Genes Dis 2:186–196. doi:10.1016/j.gendis.2015.02.007.NKCC1
Ali BH, Moundhri MS Al (2006) Agents ameliorating or augmenting the nephrotoxicity of cisplatin and other platinum compounds: a review of some recent research. Food Chem Toxicol 44:1173–1183. doi:10.1016/j.fct.2006.01.013
Sekine I, Yamada K, Nokihara H et al (2007) Bodyweight change during the first 5 days of chemotherapy as an indicator of cisplatin renal toxicity. Cancer Sci 98:1408–1412. doi:10.1111/j.1349-7006.2007.00532.x
Lajer H, Kristensen M, Hansen HH et al (2005) Magnesium depletion enhances cisplatin-induced nephrotoxicity. Cancer Chemother Pharmacol 56:535–542. doi:10.1007/s00280-005-1010-7
Li Xia, Chen Z, Su K et al (2014) Comparison of cochlear cell death caused by cisplatin, alone and in combination with furosemide. Toxicol Pathol 42:376–385. doi:10.1177/0192623313483213.Comparison
Ding D, Jiang H, Wang P, Salvi R (2007) Cell death after co-administration of cisplatin and ethacrynic acid. Hear Res 226:129–139. doi:10.1016/j.heares.2006.07.015
Jaggi AS, Kaur A, Bali A, Singh N (2015) Expanding spectrum of sodium potassium chloride co-transporters in the pathophysiology of diseases. Curr Neuropharmacol 13:369–388
Huang C, Zheng M, Yang Z et al (2008) Projection of exposure and efficacious dose prior to first-in-human studies: how successful have we been? Pharm Res 25:713–726. doi:10.1007/s11095-007-9411-4
Lallemand B, Masi M, Maddau L et al (2012) Evaluation of in vitro anticancer activity of sphaeropsidins A-C, fungal rearranged pimarane diterpenes, and semisynthetic derivatives. Phytochem Lett 5:770–775. doi:10.1016/j.phytol.2012.08.011
Rahman A, Treat J, Roh J et al (1990) A phase I clinical trial and pharmacokinetic evaluation of liposome-encapsulated doxorubicin. J Clin Oncol 8:1093–1100
Gardner ER, Dahut WL, Scripture CD et al (2008) Randomized crossover pharmacokinetic study of solvent-based paclitaxel and nab-paclitaxel. Clin Cancer Res 14:4200–4205. doi:10.1158/1078-0432.CCR-07-4592.Randomized
Acknowledgements
We thank Robert Kiss for his advice in the current project and particularly for having initiated it as well as for reviewing the present manuscript. ADG is a postdoctoral fellow of the FWO-Vlaanderen, Belgium.
Funding
No private or public grant supported this work that was conducted with academic financial support only.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ingels, A., Dinhof, C., Garg, A.D. et al. Computed determination of the in vitro optimal chemocombinations of sphaeropsidin A with chemotherapeutic agents to combat melanomas. Cancer Chemother Pharmacol 79, 971–983 (2017). https://doi.org/10.1007/s00280-017-3293-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-017-3293-x