Abstract
Purpose
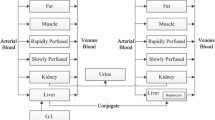
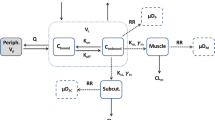
The objectives of this study were to develop physiologically based models for the pharmacokinetics (PK) and organ distribution of apicidin in rats and mice and to predict human PK in blood and organs.
Methods
The PK of apicidin was characterized in rats and mice after i.v. bolus injection, and distribution to various tissues was determined in rats following i.v. infusions at steady state. The developed models were prospectively validated within rat and within mouse and by scaling from rat to mouse using data after multiple i.v. injections. Human PK was predicted by the physiologically based modeling using intrinsic clearance data for humans from in vitro experiments.
Results
The Cls predicted for human (9.8 ml/min/kg) was lower than those found in mice (116.9 ml/min/kg) and rats (61.6 ml/min/kg), and the Vss predicted for human (1.9 l/kg) was less than in mice (2.0 l/kg) and rats (2.5 l/kg). Consequently, the predicted t 1/2 was longer in human (2.3 h) than in mice and rats (0.4 and 0.9 h, respectively). The highest concentrations of apicidin were predicted in liver followed by adipose tissue, kidney, lung, spleen, heart, arterial blood, venous blood, small intestine, stomach, muscle, testis, and brain.
Conclusions
The developed models adequately described the PK of apicidin in rats and mice and were applied to predict human PK. These models may be useful in predicting human blood and tissue concentrations of apicidin under different exposure conditions.





Similar content being viewed by others
References
Marks PA, Richon VM, Rifkind RA (2000) Histone deacetylase inhibitors: inducers of differentiation or apoptosis of transformed cells. J Natl Cancer Inst 92:1210–1216
Meinke PT, Liberator P (2001) Histone deacetylase: a target for antiproliferative and antiprotozoal agents. Curr Med Chem 8:211–235
Marks PA, Richon VM, Rifkind RA (2000) Histone deacetylase inhibitors: inducers of differentiation or apoptosis of transformed cells. J Natl Cancer Inst 92:1210–1216
Konstantinopoulos PA, Vandoros GP, Papavassiliou AG (2006) FK228 (depsipeptide): a HDAC inhibitor with pleitropic antitumor activities. Cancer Chemother Pharmacol 58:711–715
Krämer OH, Göttlicher M, Heinzel T (2001) Histone deacetylase as a therapeutic target. Trends Endocrin Metab 12:294–300
Darkin-Rattray SJ, Gurnett AM, Myers RW, Dulski PM, Crumley TM, Allocco JJ, Cannova C, Meinke PT, Colletti SL, Bednarek MA, Singh SB, Goetz MA, Dombrowski AW, Polishook JD, Schmatz DM (1996) Apicidin: a novel antiprotozoal agent that inhibits parasite histone deacetylase. Proc Natl Acad Sci USA 93:13143–13147
Han JW, Ahn SH, Park SH, Wang SY, Bae GU, Seo DW, Kwon HK, Hong S, Lee HY, Lee YW, Lee HW (2000) Apicidin, a histone deacetylase inhibitor, inhibits proliferation of tumor cells via induction of p21WAF1/Cip1 and Gelsolin1. Cancer Res 60:6068–6074
Kwon SH, Ahn SH, Kim YK, Bae GU, Yoon JW, Hong S, Lee HY, Lee YW, Lee HW, Han JW (2002) Apicidin, a histone deacetylase inhibitor, induces apoptosis and Fas/Fas ligand expression in human acute promyelocytic leukemia cells. J Biol Chem 277:2073–2080
Park H, Im JY, Kim J, Choi WS, Kim HS (2008) Effects of apicidin, a histone deacetylase inhibitor, on the regulation of apoptosis in H-ras-transformed breast epithelial cells. Int J Mol Med 21:325–333
Noh JH, Song JH, Eun JW, Kim JK, Jung KH, Bae HJ, Xie HJ, Ryu JC, Ahn YM, Wee SJ, Park WS, Lee JY, Nam SW (2009) Systemic cell-cycle suppression by apicidin, a histone deacetylase inhibitor, in MDA-MB-435 cells. Int J Mol Med 24:205–226
Kim SH, Ahn S, Han JW, Lee HW, Lee HY, Lee YW, Kim MR, Kim KW, Kim WB, Hong S (2004) Apicidin is a histone deacetylase inhibitor with anti-invasive and anti-angiogenic potentials. Biochem Biophys Res Commun 315:964–970
Pluemsampant S, Safronova OS, Nakahama K, Morita I (2008) Protein kinase CK2 is a key activator of histone deacetylase in hypoxia-associated tumors. Int J Cancer 122:333–341
Shin BS, Chang HS, Park EH, Yoon CH, Kim HY, Kim J, Ryu JK, Zee OP, Lee KC, Cao D, Yoo SD (2006) Pharmacokinetics of a novel histone deacetylase inhibitor, apicidin, in rats. Biopharm Drug Dispos 27:69–75
Shin BS, Park EH, Yoon CH, Kim J, Zee OP, Yoo SD (2005) In vitro investigation of the hepatic intrinsic clearance of apicidin, a histone deacetylase inhibitor, in mouse, rat, and human with correlation by nonspecific protein binding. J Toxicol Environ Health A 68:2207–2218
Mahmood I (2009) Pharmacokinetic allometric scaling of antibodies: application to the first-in-human dose estimation. J Pharm Sci 98:3850–3861
Karalis V, Claret L, Iliadis A, Macheras P (2001) Fractal volume of drug distribution: it scales proportionally to body mass. Pharm Res 18:1056–1060
Nestorov IA, Aarons LJ, Arundel PA, Rowland M (1998) Lumping of whole-body physiologically based pharmacokinetic models. J Pharmacokinet Biopharm 26:21–46
Meno-Tetang GML, Li H, Mis S, Pyszczynski N, Heining P, Lowe P, Jusko WJ (2006) Physiologically based pharmacokinetic modeling of FTY720 (2-amino-2[2-(-4-octylphenyl)ethyl]propane-1, 3-diol hydrochloride) in rats after oral and intravenous doses. Drug Metab Dispos 34:1480–1487
Ruelius HW (1987) Extrapolation from animals to man: predictions, pitfalls and perspectives. Xenobiotica 17:255–265
Park JS, Lee KR, Kim JC, Lim SH, Seo JA, Lee YW (1999) A hemorrhagic factor (apicidin) produced by toxic Fusarium isolates from soybean seeds. Appl Environ Microbiol 65:126–130
Shin BS, Kim J, Yoon CH, Kim CH, Park EH, Han JW, Yoo SD (2005) Development of a liquid chromatography/electrospray tandem mass spectrometry assay for the quantification of apicidin, a novel histone deacetylase inhibitor, in rat serum: application to a pharmacokinetic study. Rapid Commun Mass Spectrom 19:408–414
Bernareggi A, Rowland M (1991) Physiologic modeling of cyclosporin kinetics in rat and man. J Pharmacokinet Biopharm 19:21–50
Brown RP, Delp MD, Lindstedt SL, Rhomberg LR, Beliles RP (1997) Physiological parameter values for physiologically based pharmacokinetic models. Toxicol Ind Health 13:407–484
Davies B, Morris T (1993) Physiological parameters in laboratory animals and humans. Pharm Res 10:1093–1095
Mager DE (2006) Quantitative structure-pharmacokinetic/pharmacodynamic relationships. Adv Drug Deliv Rev 58:1326–1356
Acknowledgments
This work was supported in part by the National Research Foundation (NRF) grant (2009-0083962) and the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (No. 2010-0022780).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shin, B.S., Bulitta, J.B., Balthasar, J.P. et al. Prediction of human pharmacokinetics and tissue distribution of apicidin, a potent histone deacetylase inhibitor, by physiologically based pharmacokinetic modeling. Cancer Chemother Pharmacol 68, 465–475 (2011). https://doi.org/10.1007/s00280-010-1502-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-010-1502-y