Abstract
Purpose
The purpose of the article is to determine whether changes in apparent diffusion coefficient (ADC) values of locally advanced rectal cancer (LARC) obtained 2 weeks after the beginning of chemoradiation therapy (CRT) allow to predict treatment response and whether correlate with tumor histopathologic response.
Methods
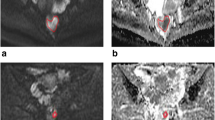
Forty-three patients receiving CRT for LARC and 3.0T magnetic resonance imaging with diffusion-weighted sequences before treatment, 2 weeks during, and 8 weeks post the completion of CRT were included. ADC values were calculated at each time point and percentage of ADC changes at 2 weeks (ΔADC during) and 8 weeks (ΔADC post) were assessed. Data were correlated to surgical results and histopathologic tumor regression grade (TRG), according to Mandard’s classification. ADC values and ΔADCs of complete responders (CR; TRG1) and non-complete responders (non-CR; TRG 2-5) were compared. Receiver-operating characteristic curve (ROC) analysis was used to assess diagnostic accuracy of ΔADC for differentiating CR from non-CR. The correlation with TRG was investigated using Spearman's rank test.
Results
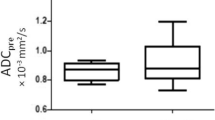
ΔADC during and ΔADC post were significantly higher in CR (33.9% and 57%, respectively) compared to non-CR (13.5% and 2.2%, respectively) group (p = 0.006 and p < 0.001, respectively). ROC analysis revealed the following diagnostic performances: ΔADC during: AUC 0.78 (0.08), p = 0.004, cut-off 20.6% (sensitivity 75% and specificity 76.5%); ΔADC post: AUC 0.94 (0.04), p ≤ 0.001, cut-off 22% (sensitivity 95% and specificity 82.4%). Significant moderate and good negative correlation was found between ΔADC during and ΔADC post and TRG (r = − 0.418, p = 0.007; r = − 694, p ≤ 0.001, respectively).
Conclusion
ΔADC at 2 weeks after the beginning of CRT is a reliable tool to early assess treatment response.






Similar content being viewed by others
References
Valentini V, Aristei C, Glimelius B, et al. (2009) Multidisciplinary rectal cancer management: 2d rectal cancer consensus conference (EURECA-CC2). Radiother Oncol 92:148–163
Park IJ, You YN, Agarwal A, et al. (2012) Neoadjuvant treatment response as an early response indicator for patients with rectal cancer. J Clin Oncol 30:1770–1776
Gollins S, Sebag-Montefiore D (2016) Neoadjuvant treatment strategies for locally advanced rectal cancer. Clin Oncol 28(2):146–151 ((R Coll Radiol))
Burbach JP, den Harder AM, Intven M, et al. (2014) Impact of radiotherapy boost on pathological complete response in patients with locally advanced rectal cancer: a systematic review and meta-analysis. Radiother Oncol 113(1):1–9
Feliu J, Calvilio J, Escribano A, et al. (2002) Neoadjuvant therapy of rectal carcinoma with UFT-leucovorin plus radiotherapy. Ann Oncol 13(5):730–736
Fernandez-Martos C, Aparicio J, Bosch C, et al. (2004) Preoperative uracil, tegafur, and concomitant radiotherapy in operable rectal cancer: a phase II multicenter study with 3 years’ follow-up. J Clin Oncol 22(15):3016–3022
Barbaro B, Fiorucci C, Tebala C, et al. (2009) Locally advanced rectal cancer: MR imaging in prediction of response after preoperative chemotherapy and radiation therapy. Radiology 250:730–739
Dzik-Jurasz A, Domenig C, George M, et al. (2002) Diffusion MRI for prediction of response of rectal cancer to chemoradiation. Lancet 360:307–308
Kim SH, Lee JM, Lee JM, Han JK, Choi BI (2011) Apparent diffusion coefficient for evaluating tumor response to neoadjuvant chemoradiation therapy for locally advanced rectal cancer. Eur Radiol 21:987–995
Jung SH, Heo SH, Kim JW, et al. (2012) Predicting response to neoadjuvant chemoradiation therapy in locally advanced rectal cancer: diffusion-weighted 3 Tesla MR imaging. J Magn Reson Imaging 35(1):110–116
Genovesi D, Filippone A, Cefaro GA, et al. (2013) Diffusion-weighted magnetic resonance for prediction of response after neoadjuvant chemoradiation therapy for locally advanced rectal cancer: preliminary results of a monoinstitutional prospective study. Eur J Surg Oncol 39:1071–1078
Lambregts DM, Vandecaveye V, Barbaro B, et al. (2011) Diffusion-weighted MRI for selection of complete responders after chemoradiation for locally advanced rectal cancer: a multicenter study. Ann Surg Oncol 18:2224–2231
Chen YG, Chen MQ, Guo YY, et al. (2016) Apparent diffusion coefficient predicts pathology complete response of rectal cancer treated with neoadjuvant chemoradiotherapy. PLoS ONE 11(4):e0153944
Monguzzi L, Ippolito D, Bernasconi DP, et al. (2013) Locally advanced rectal cancer: value of ADC mapping in prediction of tumor response to radiochemotherapy. Eur J Radiol. 82(2):234–240
Kim YC, Lim JS, Keum KC, et al. (2011) Comparison of diffusion-weighted MRI and MR volumetry in the evaluation of early treatment outcomes after preoperative chemoradiotherapy for locally advanced rectal cancer. J Magn Reson Imaging 34:570–576
Sun YS, Zhang XP, Tang L, et al. (2010) Locally advanced rectal carcinoma treated with preoperative chemoradiation therapy: preliminary analysis of diffusion-weighted MR imaging for early detection of tumor histopathologic downstaging. Radiology 254:171–178
Barbaro B, Vitale R, Valentini V, et al. (2012) Diffusion-weighted magnetic resonance imaging in monitoring rectal cancer response to neoadjuvant chemoradiotherapy. Int J Radiat Oncol Biol Phys 83(2):594–599
Lambrecht M, Vandecaveye V, De Keyzer F, et al. (2012) Value of diffusion-weighted magnetic resonance imaging for prediction and early assessment of response to neoadjuvant radiochemotherapy in rectal cancer: preliminary results. J Radiat Oncol Biol Phys 82:863–870
Jacobs L, Intven M, van Lelyveld N, et al. (2016) Diffusion-weighted MRI for early prediction of treatment response on preoperative chemoradiotherapy for patients with locally advanced rectal cancer. A feasibility study. Ann Surg 263:522–528
Mandard AM, Dalibard F, Mandard JC, et al. (1994) Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma: clinicopathologic correlations. Cancer 73:2680–2686
Humphrics PD, Sebire NJ, Siegel MJ, Olsen OE (2007) Tumors in pediatric patients at diffusion-weighted MR imaging: apparent diffusion coefficient and tumor cellularity. Radiology 245:848–854
Koh D, Collins D (2007) Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR Am J Roentgenol 188:1622–1635
DeVries AF, Kremser C, Hein PA, et al. (2003) Tumor microcirculation and diffusion predict therapy outcome for primary rectal carcinoma. Int J Radiat Oncol Biol Phys 56(4):958–965
Xie H, Sun T, Chen M, et al. (2015) Effectiveness of the apparent diffusion coefficient for predicting the response to chemoradiation therapy in locally advanced rectal cancer: a systematic review and meta-analysis. Medicine 94(6):e517
Lambregts DM, Beets GL, Maas M, et al. (2011) Tumour ADC measurements in rectal cancer: effect of ROI methods on ADC values and interobserver variability. Eur Radiol 21:2567–2574
Cai G (2012) Diffusion-weighted magnetic resonance imaging for predict- ing the response of rectal cancer to neoadjuvant concurrent chemoradiation. World J Gastroenterol 19:5520–5527
Sun YS, Zhang XP, Tang L, et al. (2010) Locally advanced rectal carcinoma treated with preoperative chemotherapy and radiation therapy: preliminary analysis of diffusion-weighted MR imaging for early detection of tumor histopathologic downstaging. Radiology 254:170–178
Kremser C, Judmaier W, Hein P, et al. (2003) Preliminary results on the influence of chemoradiation on apparent diffusion coefficients of primary rectal carcinoma measured by magnetic resonance imaging. Strahlenther Onkol 179:641–649
Bipat S, Glas AS, Slors FJ, et al. (2004) Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging—a meta-analysis. Radiology 232:773–783
Van der Paardt MP, Zager MB, Beets-Tan RG, et al. (2013) Patients who undergo preoperative chemoradiotherapy for locally advanced rectal cancer restaged by using diagnostic MR imaging: a systematic review and meta-analysis. Radiology 269(1):101–112
Zhao R-S, Wang H, Zhou Z-Y, et al. (2014) Restaging of locally advanced rectal cancer with magnetic resonance imaging and endoluminal ultrasound after preoperative chemoradiotherapy. Dis Colon Rectum. 57:388–395
Chang KJ, Kamel IR, Macura KJ, Bluemke DA (2008) 3.0-T MR imaging of the abdomen: comparison with 1.5 T. Radiographics 28:1983–1998
Erturk SM, Alberich-Bayarri A, Herrmann KA, Marti-Bonmati L, Ros PR (2009) Use of 3.0-T MR imaging for evaluation of the abdomen. Radiographics 29:1547–1563
Author information
Authors and Affiliations
Contributions
All authors were involved in patient management and wrote the report. Written consent to publication was obtained.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was approved by the local institutional review board. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Delli Pizzi, A., Cianci, R., Genovesi, D. et al. Performance of diffusion-weighted magnetic resonance imaging at 3.0T for early assessment of tumor response in locally advanced rectal cancer treated with preoperative chemoradiation therapy. Abdom Radiol 43, 2221–2230 (2018). https://doi.org/10.1007/s00261-018-1457-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-018-1457-8