Abstract
Purpose
The aim of this study was to explore the association of cardiac fibroblast activation with clinical parameters and cardiovascular magnetic resonance (CMR) imaging parameters in patients with chronic thromboembolic pulmonary hypertension (CTEPH).
Methods
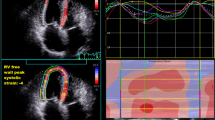
Thirteen CTEPH patients were prospectively enrolled. All of the patients underwent cardiac 68Gallium-labelled fibroblast activation protein inhibitor (68 Ga-FAPI-04)-positron emission tomography/computed tomography (PET/CT), right heart catheterisation, and echocardiography, and 11 of them additionally underwent CMR. Thirteen control subjects were selected to establish the normal range of cardiac 68 Ga-FAPI-04 uptake. Cardiac 68 Ga-FAPI-04 uptake higher than that in the blood pool was defined as abnormal. The global and segmental maximum standardised uptake values (SUVmax) of the right ventricle (RV) were measured and further expressed as target-to-background ratio (TBRRV) with left ventricular lateral wall activity as background. Late gadolinium enhancement (LGE) was visually evaluated, and native-T1 times, enhanced-T1 times, and extracellular volume (ECV) were quantitatively measured.
Results
Ten CTEPH patients (77%) had abnormal 68 Ga-FAPI-04 uptake in RV, mainly located in the free wall, which was significantly higher than that in controls (TBRRV: 2.4 ± 0.9 vs 1.0 ± 0.1, P < 0.001). The TBRRV correlated positively with the thickness of RV wall (r = 0.815, P = 0.001) and inversely with RV fraction area change (RVFAC) (r = − 0.804, P = 0.001) and tricuspid annular plane systolic excursion (TAPSE) (r = − 0.678, P = 0.011). No correlation was found between 68 Ga-FAPI-04 activity and CMR imaging parameters.
Conclusion
Fibroblast activation in CTEPH, measured by 68 Ga-FAPI-04 imaging, is mainly localised in the RV free wall. Enhanced fibroblast activation reflects the thickening of the RV wall and decreased RV contractile function.




Similar content being viewed by others
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Code availability
Not applicable.
References
van de Veerdonk MC, Bogaard HJ, Voelkel NF. The right ventricle and pulmonary hypertension. Heart Fail Rev. 2016;21(3):259–71. https://doi.org/10.1007/s10741-016-9526-y.
Delcroix M, Vonk Noordegraaf A, Fadel E, Lang I, Simonneau G, Naeije R. Vascular and right ventricular remodeling in chronic thromboembolic pulmonary hypertension. Eur Respir J. 2013;41(1):224–32. https://doi.org/10.1183/09031936.00047712.
Asosingh K, Erzurum S. Mechanisms of right heart disease in pulmonary hypertension (2017 Grover Conference Series). Pulm Circ. 2018;8(1):2045893217753121. https://doi.org/10.1177/2045893217753121.
Rain S, Handoko ML, Trip P, Gan CT, Westerhof N, Stienen GJ, Paulus WJ, Ottenheijm CA, Marcus JT, Dorfmüller P, Guignabert C, Humbert M, Macdonald P, Dos Remedios C, Postmus PE, Saripalli C, Hidalgo CG, Granzier HL, Vonk-Noordegraaf A, van der Velden J, de Man FS. Right ventricular diastolic impairment in patients with pulmonary arterial hypertension. Circulation. 2013;128(1):2016–25. https://doi.org/10.1161/CIRCULATIONAHA.113.001873.
Trip P, Rain S, Handoko ML, van der Bruggen C, Bogaard HJ, Marcus JT, Boonstra A, Westerhof N, Vonk-Noordegraaf A, de Man FS. Clinical relevance of right ventricular diastolic stiffness in pulmonary hypertension. Eur Respir J. 2015;45(6):1603–12. https://doi.org/10.1183/09031936.00156714.
Kusakari Y, Urashima T, Shimura D, Amemiya E, Miyasaka G, Yokota S, Fujimoto Y, Akaike T, Inoue T, Minamisawa S. Impairment of excitation-contraction coupling in right ventricular hypertrophied muscle with fibrosis induced by pulmonary artery banding. PLoS ONE. 2017;12(1):e0169564. https://doi.org/10.1371/journal.pone.0169564.
Rochitte CE, Tassi EM, Shiozaki AA. The emerging role of MRI in the diagnosis and management of cardiomyopathies. Curr Cardiol Rep. 2006;8(1):44–52. https://doi.org/10.1007/s11886-006-0010-5.
Flett AS, Hayward MP, Ashworth MT, Hansen MS, Taylor AM, Elliott PM, McGregor C, Moon JC. Equilibrium contrast cardiovascular magnetic resonance for the measurement of diffuse myocardial fibrosis. Circulation. 2010;122(2):138–44. https://doi.org/10.1161/CIRCULATIONAHA.109.930636.
Disertori M, Rigoni M, Pace N, Casolo G, Masè M, Gonzini L, Lucci D, Nollo G, Ravelli F. Myocardial fibrosis assessment by LGE is a powerful predictor of ventricular tachyarrhythmias in ischemic and nonischemic LV dysfunction: a meta-analysis. JACC Cardiovasc Imaging. 2016;9(9):1046–55. https://doi.org/10.1016/j.jcmg.2016.01.033.
Dass S, Suttie JJ, Piechnik SK, Ferreira VM, Holloway CJ, Banerjee R, Mahmod M, Cochlin L, Karamitsos TD, Robson MD, Watkins H, Neubauer S. Myocardial tissue characterization using magnetic resonance noncontrast t1 mapping in hypertrophic and dilated cardiomyopathy. Circ Cardiovasc Imaging. 2012;5(6):726–33. https://doi.org/10.1161/CIRCIMAGING.112.976738.
Alabed S, Saunders L, Garg P, Shahin Y, Alandejani F, Rolf A, Puntmann VO, Nagel E, Wild JM, Kiely DG, Swift AJ. Myocardial T1-mapping and extracellular volume in pulmonary arterial hypertension: a systematic review and meta-analysis. Magn Reson Imaging. 2021;79:66–75. https://doi.org/10.1016/j.mri.2021.03.011.
Krenning G, Zeisberg EM, Kalluri R. The origin of fibroblasts and mechanism of cardiac fibrosis. J Cell Physiol. 2010;225(3):631–7. https://doi.org/10.1002/jcp.22322.
Souders CA, Bowers SLK, Baudino TA. Cardiac fibroblast: the renaissance cell. Circ Res. 2009;105(12):1164–76. https://doi.org/10.1161/CIRCRESAHA.109.209809.
Blankesteijn WM. Has the search for a marker of activated fibroblasts finally come to an end? J Mol Cell Cardiol. 2015;88:120–3. https://doi.org/10.1016/j.yjmcc.2015.10.005.
Tillmanns J, Hoffmann D, Habbaba Y, Schmitto JD, Sedding D, Fraccarollo D, Galuppo P, Bauersachs J. Fibroblast activation protein alpha expression identifies activated fibroblasts after myocardial infarction. J Mol Cell Cardiol. 2015;87:194–203. https://doi.org/10.1016/j.yjmcc.2015.08.016.
Park JE, Lenter MC, Zimmermann RN, Garin-Chesa P, Old LJ, Rettig WJ. Fibroblast activation protein, a dual specificity serine protease expressed in reactive human tumor stromal fibroblasts. J Biol Chem. 1999;274(51):36505–12. https://doi.org/10.1074/jbc.274.51.36505.
Rog-Zielinska EA, Norris RA, Kohl P, Markwald R. The living scar–cardiac fibroblasts and the injured heart. Trends Mol Med. 2016;22(2):99–114. https://doi.org/10.1016/j.molmed.2015.12.006.
de Haas HJ, van den Borne SW, Boersma HH, Slart RH, Fuster V, Narula J. Evolving role of molecular imaging for new understanding: targeting myofibroblasts to predict remodeling. Ann N Y Acad Sci. 2012;1254(1):33–41. https://doi.org/10.1111/j.1749-6632.2012.06476.x.
Varasteh Z, Mohanta S, Robu S, Braeuer M, Li Y, Omidvari N, Topping G, Sun T, Nekolla SG, Richter A, Weber C, Habenicht A, Haberkorn UA, Weber WA. Molecular imaging of fibroblast activity after myocardial infarction using a 68Ga-labeled fibroblast activation protein inhibitor, FAPI-04. J Nucl Med. 2019;60(12):1743–9. https://doi.org/10.2967/jnumed.119.226993.
Shi X, Lin X, Huo L, Li X. Cardiac fibroblast activation in dilated cardiomyopathy detected by positron emission tomography. J Nucl Cardiol. 2020. https://doi.org/10.1007/s12350-020-02315-w.
Wang L, Zhang Z, Zhao Z, Yan C, Fang W. 68Ga-FAPI right heart uptake in a patient with idiopathic pulmonary arterial hypertension. J Nucl Cardiol. 2020. https://doi.org/10.1007/s12350-020-02407-7.
Xing HQ, Gong JN, Chen BX, Guo XJ, Yang YH, Huo L, Yang MF. Comparison of 68Ga-FAPI imaging and cardiac magnetic resonance in detection of myocardial fibrosis in a patient with chronic thromboembolic pulmonary hypertension. J Nucl Cardiol. 2021. https://doi.org/10.1007/s12350-020-02517-2.
Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Rev Esp Cardiol (Engl Ed). 2016;69(2):177. https://doi.org/10.1016/j.rec.2016.01.002.
Lindner T, Loktev A, Altmann A, Giesel F, Kratochwil C, Debus J, Jäger D, Mier W, Haberkorn U. Development of quinoline-based theranostic ligands for the targeting of fibroblast activation protein. J Nucl Med. 2018;59(9):1415–22. https://doi.org/10.2967/jnumed.118.210443.
Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography: endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23(7):685–713. https://doi.org/10.1016/j.echo.2010.05.010.
Leslie KO, Taatjes DJ, Schwarz J, vonTurkovich M, Low RB. Cardiac myofibroblasts express alpha smooth muscle actin during right ventricular pressure overload in the rabbit. Am J Pathol. 1991;139(1):207–16.
Mauritz GJ, Kind T, Marcus JT, Bogaard HJ, van de Veerdonk M, Postmus PE, Boonstra A, Westerhof N, Vonk-Noordegraaf A. Progressive changes in right ventricular geometric shortening and long-term survival in pulmonary arterial hypertension. Chest. 2012;141(4):935–43. https://doi.org/10.1378/chest.10-3277.
Forfia PR, Fisher MR, Mathai SC, Housten-Harris T, Hemnes AR, Borlaug BA, Chamera E, Corretti MC, Champion HC, Abraham TP, Girgis RE, Hassoun PM. Tricuspid annular displacement predicts survival in pulmonary hypertension. Am J Respir Crit Care Med. 2006;174(9):1034–41. https://doi.org/10.1164/rccm.200604-547OC.
Andersen S, Nielsen-Kudsk JE, Vonk Noordegraaf A, de Man FS. Right ventricular fibrosis. Circulation. 2019;139(2):269–85. https://doi.org/10.1161/CIRCULATIONAHA.118.035326.
Eghbali M. Cardiac fibroblasts: function, regulation of gene expression, and phenotypic modulation. Basic Res Cardiol. 1992;87(Suppl 2):183–9. https://doi.org/10.1007/978-3-642-72477-0_16.
Gomez-Arroyo J, Sakagami M, Syed AA, Farkas L, Van Tassell B, Kraskauskas D, Mizuno S, Abbate A, Bogaard HJ, Byron PR, Voelkel NF. Iloprost reverses established fibrosis in experimental right ventricular failure. Eur Respir J. 2015;45(2):449–62. https://doi.org/10.1183/09031936.00188013.
Friedberg MK, Cho MY, Li J, Assad RS, Sun M, Rohailla S, Honjo O, Apitz C, Redington AN. Adverse biventricular remodeling in isolated right ventricular hypertension is mediated by increased transforming growth factor-β1 signaling and is abrogated by angiotensin receptor blockade. Am J Respir Cell Mol Biol. 2013;49(6):1019–28. https://doi.org/10.1165/rcmb.2013-0149OC.
Mehta BB, Auger DA, Gonzalez JA, Workman V, Chen X, Chow K, Stump CJ, Mazimba S, Kennedy JL, Gay E, Salerno M, Kramer CM, Epstein FH, Bilchick KC. Detection of elevated right ventricular extracellular volume in pulmonary hypertension using Accelerated and Navigator-Gated Look-Locker Imaging for Cardiac T1 Estimation (ANGIE) cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2015;17:110. https://doi.org/10.1186/s12968-015-0209-y.
Wang J, Zhao H, Wang Y, Herrmann HC, Witschey WRT, Han Y. Native T1 and T2 mapping by cardiovascular magnetic resonance imaging in pressure overloaded left and right heart diseases. J Thorac Dis. 2018;10(5):2968–75. https://doi.org/10.21037/jtd.2018.04.141.
Patel RB, Li E, Benefield BC, Swat SA, Polsinelli VB, Carr JC, Shah SJ, Markl M, Collins JD, Freed BH. Diffuse right ventricular fibrosis in heart failure with preserved ejection fraction and pulmonary hypertension. ESC Heart Fail. 2020;7(1):253–63. https://doi.org/10.1002/ehf2.12565.
Bradlow WM, Assomull R, Kilner PJ, Gibbs JS, Sheppard MN, Mohiaddin RH. Understanding late gadolinium enhancement in pulmonary hypertension. Circ Cardiovasc Imaging. 2010;3(4):501–3. https://doi.org/10.1161/CIRCIMAGING.109.919779.
Marcus JT, Vonk Noordegraaf A, Roeleveld RJ, Postmus PE, Heethaar RM, Van Rossum AC, Boonstra A. Impaired left ventricular filling due to right ventricular pressure overload in primary pulmonary hypertension: noninvasive monitoring using MRI. Chest. 2001;119(6):1761–5. https://doi.org/10.1378/chest.119.6.1761.
Funding
This work was supported by Beijing Hospitals Authority Clinical Medicine Development of Special Funding Support (ZYLX202105) and the National Natural Science Foundation of China (81871380 and 82071967).
Author information
Authors and Affiliations
Contributions
Bi-Xi Chen wrote the draft of the manuscript; Bi-Xi Chen and Juan-Ni Gong collected and analysed the clinical data; Hai-Qun Xing and Xiao-Ying Xi analysed the PET/CT data; Bi-Xi Chen and Xiao-Juan Guo analysed the CMR data; Min-Fu Yang, Yuan-Hua Yang, and Li Huo conceived the study and interpreted the results; Min-Fu Yang makes critical revision of the manuscript for important intellectual content. All authors contributed to the article’s revision, agreed to its submission, and had full access to original data.
Corresponding author
Ethics declarations
Ethics approval
All procedures involving human participants were carried out in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Patients signed informed consent regarding publishing their data and photographs.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Bi-Xi Chen, Hai-Qun Xing, and Juan-Ni Gong contributed equally to this work and are co-first authors.
This article is part of the Topical Collection on Cardiology
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, BX., Xing, HQ., Gong, JN. et al. Imaging of cardiac fibroblast activation in patients with chronic thromboembolic pulmonary hypertension. Eur J Nucl Med Mol Imaging 49, 1211–1222 (2022). https://doi.org/10.1007/s00259-021-05577-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-021-05577-9