Abstract
Objective
To identify abnormalities in asymptomatic sedentary individuals using 3.0 Tesla high-resolution MRI.
Materials and methods
The cohort comprised of 230 knees of 115 uninjured sedentary adults (51 males, 64 females; median age: 44 years). All participants had bilateral knee 3.0 T MRIs. Two senior musculoskeletal radiologists graded all intraarticular knee structures using validated scoring systems. Participants completed Knee Injury and Osteoarthritis Outcome Score questionnaires at the time of the MRI scan.
Results
MRI showed abnormalities in the majority (97%) of knees. Thirty percent knees had meniscal tears: horizontal (23%), complex (3%), vertical (2%), radial (2%) and bucket handle (1%). Cartilage and bone marrow abnormalities were prevalent at the patellofemoral joint (57% knees and 48% knees, respectively). Moderate and severe cartilage lesions were common, in 19% and 31% knees, respectively, while moderate and severe bone marrow oedema in 19% and 31% knees, respectively. Moderate-intensity lesion in tendons was found in 21% knees and high-grade tendonitis in 6% knees—the patellar (11% and 2%, respectively) and quadriceps (7% and 2%, respectively) tendons being most affected. Three percent partial ligamentous ruptures were found, especially of the anterior cruciate ligament (2%).
Conclusion
Nearly all knees of asymptomatic adults showed abnormalities in at least one knee structure on MRI. Meniscal tears, cartilage and bone marrow lesions of the patellofemoral joint were the most common pathological findings. Bucket handle and complex meniscal tears were reported for the first time in asymptomatic knees.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pathologies of the knee joint increase with age, and may be already existing on magnetic resonance imaging (MRI) before middle age, even without symptoms [1].
In fact, both well and poorly functioning knees can have similar damage, making it difficult to correlate relevant MRI findings with the patients’ knee pain [2,3,4]. Advice on permitted load and stress limits in asymptomatic knee pathologies to prevent from advancing osteoarthritis (OA) remain unclear [1].
MRI has high sensitivity for the detection of subtle changes of joint structures [5, 6]. The estimated prevalence of MRI lesions in asymptomatic knees varies significantly between studies, from 0 to 75% [2, 3]. This is due to varying study designs, including different MRI field strengths and sequences employed—indicative of variation in diagnostic accuracy [7, 8]—as well as cohorts of varying size and levels of physical activity [1].
Although 1.5 T MRI is widely clinically used, limitations have been acknowledged, particularly in evaluating abnormalities of the hyaline articular cartilage and meniscus [9,10,11]. Existing literature demonstrates that 3.0 T MRI provides important clinical benefits over 1.5 T, as the stronger field strength increases signal-to-noise ratio allowing improved visualisation of anatomical and pathological structures [5, 12]. Additionally, using a multichannel coil improves sensitivity and diagnostic quality [13, 14].
The purpose of this study was to determine the prevalence of abnormal knee findings in asymptomatic adults by means of a high-field strength 3.0 Tesla (T) MRI and multichannel knee coil. This is the largest study to date using this high-resolution technology to provide a robust analysis of all knee structures.
Methods
Study design and participants
This was a prospective cohort study including asymptomatic adults. The study received ethical approval and all volunteers provided written informed consent before participation.
We recruited 115 asymptomatic volunteers (51 males, 64 females; median age: 44 years, range 25–73 years). The study was London-based and the volunteers were 95% Welsh/English/Scottish/Northern Irish/British, of white ethnicity. Twenty-five volunteers were aged < 40 years and 90 were aged ≥ 40 years. The median body mass index (BMI) was 25 (19.6–38.1) kg/m2 and physical activity of low intensity was 2 (0–4) h/week. The main inclusion criteria were sedentary individuals, not meeting physical activity requirements of 30 min of moderate-intensity physical activity, 5 days/week, or 20 min of more intense physical activities, 3 days/week, based on existing health recommendations [15,16,17]; no present or previous history of knee injury; no prior knee surgery and asymptomatic knee joints. Pregnant women, individuals aged < 18 years, non-sedentary, with known knee problems or poor cardiovascular health were excluded from the study.
The participants were asked to complete a questionnaire called The Knee Injury and Osteoarthritis Outcome Score (KOOS) to assess their perceived knee condition and ensure that they were asymptomatic [18].
MRI protocol
All volunteers underwent bilateral knee 3 Tesla MR (Prisma, Siemens Healthcare, Erlangen, Germany) with a dedicated 15-channel knee coil. The imaging protocol included 3 proton density–weighted fat-suppressed (PD FS) sequences in axial (repetition time/echo time [ms]: 4630/37), sagittal (4200/41) and coronal planes (5240/41). All slices were 3 mm thick, with an image size/acquisition matrix of 320 × 320 pixels. The scanning time per volunteer was 25 min in total (to scan both knees of each volunteer).
Imaging analysis
All MR images were reviewed using a picture archiving and communications system (PACS) workstation by a senior musculoskeletal radiologist with 10-years’ experience at consultant level. Twenty percent of the cohort were randomly selected for an additional independent evaluation by a second musculoskeletal radiologist with 9-years’ experience at a consultant level.
In case of discrepancies between the radiologists’ reports concerning the findings, agreement (consensus scores) was achieved by radiologists with a consensus reading in a second MRI reporting session.
MRI findings of the knee joint were analysed using different validated scoring systems for the presence of any signal changes/lesions of varying severity for the following structures: menisci, cartilage, bone marrow, tendons, ligaments (Table 1) [3, 19,20,21,22,23,24]. Other findings were also specified, including effusion, synovial collections (prepatellar bursitis, pes anserine bursitis, Hoffa’s synovitis) and cysts (Baker’s cyst, other ganglion cysts; Table 1) [25, 26]. The scoring systems are summarised in Appendix 1 (Supplementary Materials). The patella was divided anatomically into medial and lateral regions, with the ridge being considered as part of the medial region. The tibia was divided into medial and lateral regions. The femur was divided into medial, lateral and trochlea regions and the trochlea was further divided into medial, central and lateral. The medial and lateral menisci were each divided into subregions: anterior horn and posterior horn. Scores were assigned for each individual region. All MRI abnormalities with a grade/score > 0 were counted.
Statistical analysis
Comparisons between groups were performed using the unpaired t test, Mann–Whitney U test or chi-squared test respectively. Possible associations were explored by calculating odds ratios (OR) with 95% confidence intervals (CI). Statistical significance was defined as p < 0.05 (GraphPad Prism, version 6.0c).
Results
Nearly all knees (227/230; [97%]) of asymptomatic individuals showed abnormalities in at least one of the knee structures on MRI, of varying grades of severity. These findings included meniscal tears, cartilage abnormalities, bone marrow oedema and tendon and ligament abnormalities. No major discrepancies between the scores of the two radiologists were reported. Mean KOOS scores for each individual item were ≥ 90/100: symptoms (90.0 ± 14.0); pain (94.9 ± 8.8); function in daily living (97.1 ± 6.5); function in sport and recreation (92.3 ± 11.6) and knee-related quality of life (90.4 ± 13.8). Further details are presented in Appendices 2 and 3 (Supplementary Materials).
Meniscal tears: prevalence, location, type
The prevalence of asymptomatic meniscal tears was 30% in knees (Table 2). Meniscal degeneration was present in a further 18%.
The majority of tears were located in the medial meniscus (93%), and in its posterior horn (91%; Table 2). Lateral meniscal tears were equally found in both the posterior and anterior horns.
The types of meniscal tears that we found were horizontal (23% knees), complex (3%), vertical (2%), radial (2%) and bucket handle tears (1%); meniscal extrusion was present in 3% knees (Table 2, Fig. 1).
Articular cartilage abnormalities: prevalence, severity, location
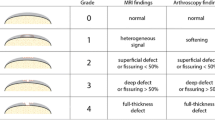
Cartilage abnormalities were present in 62% of the scanned knees (Table 3). The severity of cartilage defects were as follows: 20% knees had minor grade 1 cartilage lesions, 19% knees had grade 2, 19% knees grade 3 (moderate) cartilage lesions, 31% knees grade 4 (severe) cartilage lesions (Fig. 2); 41% knees had grade 3 and/or 4 lesions (moderate/severe).
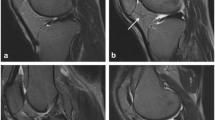
Axial proton-density fat-saturated MR images (a, c), coronal (b) and sagittal images (d) of high-grade bone marrow oedema lesion (grade 3: diameter ≥ 20 mm; in the (a) patella of the left knee of a 40-year-old man, (b) tibia of the right knee of a 59-year-old man; arrowheads) and high-grade cartilage defect (grade 4: full thickness defect exposing the bone; in the (c) patella of the left knee of a 44-year-old woman; arrow; with subchondral bone marrow oedema, arrowhead; (d) femur of the right knee of a 31-year-old woman; arrow; with subchondral ganglion cyst; small arrowhead)
The patellofemoral compartment was the most affected region (57% knees).
Bone marrow oedema: prevalence, severity, location
Bone marrow oedema–like lesions were found in 52% of the scanned knees (Table 3). By looking at levels of severity, 18% knees had only minor grade 1 bone marrow oedema lesions, 25% knees had grade 2 (moderate) oedema lesions, 7% knees had grade 3 (severe) lesions (Fig. 2) and 27% knees had grade 2 and/or 3 lesions (moderate/severe).
The region presenting with the majority of MRI changes was the patellofemoral compartment (43% knees).
Tendon abnormalities: prevalence, severity, location
We identified 46% knees with tendon abnormalities (Table 4). In terms of levels of severity, 22% knees had only minor increased signal intensity (grade 1), 21% knees had grade 2 moderate signal intensity lesions and 6% knees had grade 3 lesions/high-grade tendonitis (Fig. 3). MRI signal changes were most visible in the patellar tendon (27% knees), followed by the quadriceps tendon (13% knees).
Axial proton-density fat-saturated MR images of (a) patellar tendons (a.0, grade 0; in the left knee of a 40-year-old man; a.1, grade 1; in the right knee of a 62-year-old man; a.2, grade 2; in the left knee of a 56-year-old man; a.3, grade 3; in the right knee of a 44-year-old man) and (b) quadriceps tendons (b.0, grade 0; left knee of a 40-year-old man; b.1, grade 1; in the right knee of a 40-year-old woman; b.2, grade 2; in the left knee of a 44-year-old man; b.3, grade 3; in the right knee of a 48-year-old man). The tendons are indicated by red arrows or circles; grade 0: normal tendon appearances; grade 1: increased signal intensity in less than 25% of the axial cross-sectional tendon width; grade 2: increased high-signal intensity in 25 to 50% of the axial cross-sectional tendon width; grade 3: increased high-signal intensity occupying more than 50% of the axial cross-sectional tendon width
Ligamentous abnormalities: prevalence, severity, location
We found 38% knees (Table 4) with ligamentous abnormalities. In terms of levels of severity, 35% knees had only a thickened ligament (grade 1) and 3% knees had grade 2/partial rupture. No grade 3 injuries were identified.
The anterior cruciate ligament was the most affected ligament among the participants (34% knees), with the other ligaments presenting only very few lesions (Table 4).
Prevalence of other findings
Joint effusion was found in 3% knees: grade 2 (n = 7) and grade 3 (n = 1).
Other findings included Baker’s cyst (33% knees), prepatellar bursitis (26% knees), Hoffa’s synovitis (23% knees), other ganglion cysts (20% knees) and pes anserine bursitis (6% knees).
Associations between lesions
There was an association between the presence of abnormal cartilage signal and bone marrow oedema in knees (p < 0.0001). Participants with cartilage abnormalities were 8.0 times more likely to have bone marrow oedema lesion (95% CI, 1.6–10.3; p = 0.0023). No associations were found for other lesions (p > 0.005; Appendix 4 (Supplementary Materials)).
Participant characteristics
No difference in the prevalence of MRI abnormalities between males and females was found.
The prevalence of lesions generally increased with age. The mean age for the participants with a meniscal tear was slightly higher than those without a tear (47.5 ± 9.9 years (n = 50) vs 42.6 ± 7.0 (n = 65); p = p = 0.0027, unpaired t test). The mean age for those with bone marrow oedema was slightly higher than those without oedema (46.4 ± 8.9 years (n = 72) vs 42.0 ± 7.8 (n = 43); p = p = 0.0071, unpaired t test). Participants aged ≥ 40 years old were 4.0 times more likely to have abnormal cartilage signal (95% CI, 1.6–10.3; p = 0.0023). In terms of level of severity, 51 of 90 participants (57%) aged ≥ 40 years had high grade 3 or 4 cartilage lesions. And 10 of 25 participants (40%) aged < 40 had grade 3 or 4 cartilage lesion. The difference was not statistically significant (p = 0.140, chi-squared). The distribution of prevalences per knees is available in Table 5.
The BMI of participants with MRI abnormalities was not significantly different from those without abnormalities, except for tendon abnormalities (p = 0.0002). The odds of a participant with BMI ≥ 25 kg/m2 (overweight) presenting with a tendon abnormality were 3.3 (95% CI, 1.5–7.6). A total of 28 of 60 participants (47%) with BMI ≥ 25 kg/m2 had grade 2 or 3 high-intensity tendonitis (Fig. 3); 18 of 55 participants (33%) with BMI < 25 kg/m2 showed high-grade tendon lesion (the difference was not statistically significant, p = 0.128, chi-squared).
Discussion
Overall our study showed a high prevalence of 3.0 T MRI pathologies in the knees of asymptomatic adults: meniscal tears, including few complex and bucket handle tears; patellofemoral cartilage lesions and bone marrow oedema lesions of moderate to severe grade. The prevalences were higher than in previous studies. The KOOS results confirmed that the participants had no perceived knee problems/symptoms of functional limitation, despite the observed lesions on MRI.
Previous studies in asymptomatic uninjured knees
A number of studies have reported prevalences of knee abnormalities in uninjured asymptomatic individuals. Culvenor et al. [1] collated in a recent systematic review the pooled results from the existing evidence.
The first interesting finding is the prevalence of meniscal tears. While 44 studies (3761 knees from 2817 participants) reported prevalence of meniscal tears with an overall pooled prevalence estimate of 10% (95% CI 7 to 13%; I2 = 87.2%) [1], we hereby reported a significantly higher prevalence of 30%. Moreover, we identified vertical, radial, bucket handle and complex tears which are not common in asymptomatic individuals [27]. Therefore, they may be clinically more meaningful.
In terms of cartilage defects (partial and full thickness), 42 studies (4322 knees from 3446 participants) reported an overall pooled prevalence estimate of 24% (95% CI 15 to 34%; I2 = 97.8%) [1]. Our study however showed a higher prevalence that exceeds this interval: 41% cartilage defects of moderate to severe damage, with grade 4 lesions being most prevalent in asymptomatic adults (31% knees). The clinical significance of this is uncertain, raising questions about the factors leading to cartilage damage and what mechanisms of pathology prevention could be employed.
Thirty-four studies (4089 knees from 3255 participants) reported bone marrow lesions prevalence with an overall pooled prevalence estimate of 18% (95% CI 12 to 24%) [1]. In comparison with this data, our study showed a slightly higher prevalence of 27% moderate to severe bone marrow oedema–like lesions. Clinically, this may be of importance as bone marrow lesions are linked to the onset of osteoarthritis [28,29,30].
Prevalence of ligament tears was 0% for 16 of the 20 studies, with the remaining four studies reporting 1–30% of mostly anterior cruciate or collateral ligament partial tears [1]. Similarly, our results showed no complete tears and a low prevalence of 3% partial ligamentous tears, of the anterior cruciate and lateral collateral ligaments.
Regarding asymptomatic knee tendon abnormalities, there is not much evidence in the literature about their incidence. Matiotti SB et al. [31] identified 19.5% tendon injuries in asymptomatic soccer players—adolescents—and we identified a prevalence of 26% cases of tendon abnormalities in our study. The observation of asymptomatic patellar tendonitis may suggest that this type of injury could result in future symptoms future and encourages closer monitoring of these cases [31,32,33,34].
The prevalence of lesions was reported to increase with age [1]; this is in agreement with our study outcomes. Also we showed that overweight people are more predisposed to load-bearing tendon thickness, finding which is supported by previous studies [35,36,37,38,39].
Study strengths and limitations
The main study strengths are the large sample size, the methodology employed in the study (3.0 T MRI and multichannel coil) and the detailed analysis of knee structures. As compared with the clinically widely used 1.5 T system, 3.0 T MRI reported higher diagnostic confidence for better visualisation of the morphology and pathology of joint structures [5, 6, 40]. Also, the multichannel technology offers additional benefits of higher spatial resolution and increased diagnostic quality [13, 14]. So far 11 studies have employed the 3.0 T MRI technique for the assessment of knee structures and the sample size did not exceed 95 asymptomatic knees in any MRI trial [41,42,43,44,45,46,47,48,49,50,51]. This study involves the highest number of knees that were ever scanned with 3.0 T MRI, in particular of asymptomatic sedentary older adults. Additionally, we did an in-depth analysis of all structures and reported the prevalence of lesions by levels of severity instead of reporting only the abnormalities irrespective of grade.
We acknowledge the following limitations: (1) MRI double-reporting was done for 20% of the cohort; however, no major discrepancies between the radiologists’ reports were identified in this subset of images so the single-reporting of the remaining scans was considered to be reliable; (2) the KOOS questionnaires, the history of any past joint problems and the activity levels of volunteers were self-reported; therefore, a risk of bias needs to be considered; (3) the analysis was confined to one ethnic group, thus limiting the potential generalisation of the findings; (4) meniscal assessment included both meniscal horns, except for the body; therefore, few lesions could have been missed; (5) follow-up studies are needed to investigate the clinical relevance of the findings over time.
Conclusions and clinical significance
Our study questions clinical decision-making regarding arthroscopy and its efficacy in reducing symptoms and treatment. The high rate of asymptomatic adults with knee joint abnormalities on MRI may indicate why arthroscopy and other surgical interventions for these do not result in better outcomes than sham surgery [1, 52]. For example, there is no evidence to suggest that meniscectomy benefits patients presenting with meniscal tear symptoms more than sham surgery does [53]. Moreover, meniscectomy and other surgical interventions could lead to further complications or deterioration of the articular cartilage and increase the risk of osteoarthritis [54,55,56].
Despite the increasing use of high-resolution MRI, in practice, diagnosis should be primarily based on patient’s medical history and physical examination by an experienced clinician, instead of solely focusing on the MRI results. The images may assist in correlating clinical signs and symptoms but should not replace clinical evaluation [57, 58].
Our MRI findings can represent early signs of osteoarthritis and the clinical implications need to be investigated further, including follow-up studies over time, to inform efforts to diagnose and treat knee problems across the lifespan. Further studies could monitor whether the knee condition of those participants with lesions will progress at a faster rate over time than that of those without abnormalities. The findings may guide closer surveillance and prevent future injuries.
References
Culvenor AG, Øiestad BE, Hart HF, Stefanik JJ, Guermazi A, Crossley KM. Prevalence of knee osteoarthritis features on magnetic resonance imaging in asymptomatic uninjured adults: a systematic review and meta-analysis. Br J Sports Med. 2018.
Beattie KA, Boullos P, Pui M, O’Neill J, Inglis D, Webber CE, et al. Abnormalities identified in the knees of asymptomatic volunteers using peripheral magnetic resonance imaging. Osteoarthr Cartil. 2005;13(3):181–6.
Pappas GP, Vogelsong MA, Staroswiecki E, Gold GE, Safran MR. Magnetic resonance imaging of asymptomatic knees in collegiate basketball players: the effect of one season of play. Clin J Sport Med. 2016;26(6):483–9.
Guymer E, Baranyay F, Wluka AE, Hanna F, Bell RJ, Davis SR, et al. A study of the prevalence and associations of subchondral bone marrow lesions in the knees of healthy, middle-aged women. Osteoarthr Cartil. 2007;15(12):1437–42.
Wong S, Steinbach L, Zhao J, Stehling C, Ma CB, Link TM. Comparative study of imaging at 3.0 T versus 1.5 T of the knee. Skelet Radiol. 2009;38:761–9.
Fischbach F, Bruhn H, Unterhauser F, Ricke J, Wieners G, Felix R, et al. Magnetic resonance imaging of hyaline cartilage defects at 1.5T and 3.0T: comparison of medium T2-weighted fast spin echo, T1-weighted two-dimensional and three-dimensional gradient echo pulse sequences. Acta Radiol. 2005;46:67–73.
Crema MD, Roemer FW, Marra MD, Burstein D, Gold GE, Eckstein F, et al. Articular cartilage in the knee: current MR imaging techniques and applications in clinical practice and research. RadioGraphics. 2011;31(1):37–61.
Guermazi A, Roemer FW, Haugen IK, Crema MD, Hayashi D. MRI-based semiquantitative scoring of joint pathology in osteoarthritis. Nat Rev Rheumatol. 2013;9(4):236–51.
De Smet AA, Mukherjee R. Clinical, MRI, and arthroscopic findings associated with failure to diagnose a lateral meniscal tear on knee MRI. Am J Roentgenol. 2008;190(1):22–6.
Huysse WCJ, Verstraete KL. Health technology assessment of magnetic resonance imaging of the knee. Eur J Radiol. 2008;65(2):190–3.
Link TM, Stahl R, Woertler K. Cartilage imaging: motivation, techniques, current and future significance. Eur Radiol. 2007;17(5):1135–46.
Craig JG, Go L, Blechinger J, Hearshen D, Bouffard JA, Diamond M, et al. Three-tesla imaging of the knee: initial experience. Skelet Radiol. 2005;34(8):453–61.
Vernickel P, Röschmann P, Findeklee C, Lüdeke KM, Leussler C, Overweg J, et al. Eight-channel transmit/receive body MRI coil at 3T. Magn Reson Med. 2007.
Mekle R, Van Der Zwaag W, Joosten A, Gruetter R. Comparison of three commercially available radio frequency coils for human brain imaging at 3 Tesla. MAGMA. 2008;21(1-2):53–61.
Haskell WL, Lee I-M, Pate RR, Powell KE, Blair SN, Franklin BA, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39:1423–34.
World Health Organization. Global recommendations on physical activity for health. Geneva: World Heal Organ; 2010.
Knight JA. Physical inactivity: associated diseases and disorders. Ann Clin Lab Sci. 2012;42(3):320–37.
Roos EM, Lohmander LS. The Knee injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health Qual Life Outcomes. 2003;1:64.
Hunter DJ, Lo GH, Gale D, Grainger AJ, Guermazi A, Conaghan PG. The reliability of a new scoring system for knee osteoarthritis MRI and the validity of bone marrow lesion assessment: BLOKS (Boston-Leeds Osteoarthritis Knee Score). Ann Rheum Dis. 2008;67:206–11.
Roemer FW, Frobell R, Lohmander LS, Niu J, Guermazi A. Anterior Cruciate Ligament Osteoarthritis Score (ACLOAS): longitudinal MRI-based whole joint assessment of anterior cruciate ligament injury. Osteoarthr Cartil. 2014;22:668–82.
Noyes FR, Stabler CL. A system for grading articular cartilage lesions at arthroscopy. Am J Sports Med. 1989;17:505–13.
Gold GE, Chen CA, Koo S, Hargreaves BA, Bangerter NK. Recent advances in MRI of articular cartilage. Am J Roentgenol. 2009;193(3):628–38.
Kornaat PR, Ceulemans RYT, Kroon HM, Riyazi N, Kloppenburg M, Carter WO, et al. MRI assessment of knee osteoarthritis: Knee Osteoarthritis Scoring System (KOSS) - inter-observer and intra-observer reproducibility of a compartment-based scoring system. Skelet Radiol. 2005;34:95–102.
Johnson DP, Wakeley CJ, Watt I. Magnetic resonance imaging of patellar tendonitis. J Bone Jt Surg Ser B. 1996;78(3):452–7.
Peterfy CG, Guermazi A, Zaim S, Tirman PFJ, Miaux Y, White D, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthr Cartil. 2004;12:177–90.
Hunter DJ, Guermazi A, Lo GH, Grainger AJ, Conaghan PG, Boudreau RM, et al. Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI Osteoarthritis Knee Score). Osteoarthr Cartil. 2011;19:990–1002.
Zanetti M, Pfirrmann CWA, Schmid MR, Romero J, Seifert B, Hodler J. Patients with suspected meniscal tears: prevalence of abnormalities seen on MRI of 100 symptomatic and 100 contralateral asymptomatic knees. Am J Roentgenol. 2003;181(3):635–41.
Link TM, Li X. Bone marrow changes in osteoarthritis. Semin Musculoskelet Radiol. 2011:238–46.
Sudoł-Szopińska I, Kontny E, Maśliński W, Prochorec-Sobieszek M, Warczyńska A, Kwiatkowska B. Significance of bone marrow edema in pathogenesis of rheumatoid arthritis. Pol J Radiol. 2013;78:57–63.
Li G, Yin J, Gao J, Cheng TS, Pavlos NJ, Zhang C, et al. Subchondral bone in osteoarthritis: insight into risk factors and microstructural changes. Arthritis Res Ther. 2013;15(6):223.
Matiotti SB, Soder RB, Becker RG, Santos FS, Baldisserotto M. MRI of the knees in asymptomatic adolescent soccer players: a case–control study. J Magn Reson Imaging. 2017;45(1):59–65.
Cook JL, Khan KM, Kiss ZS, Purdam CR, Griffiths L. Prospective imaging study of asymptomatic patellar tendinopathy in elite junior basketball players. J Ultrasound Med. 2000;19(7):473–9.
Hagglund M, Walden M, Zwerver J, Ekstrand J. Epidemiology of patellar tendon injury in elite male soccer players. Br J Sports Med. 2011;39(9):1906–11.
Major NM, Helms CA. MR imaging of the knee: findings in asymptomatic collegiate basketball players. Am J Roentgenol. 2002;179:641–4.
Abate M. How obesity modifies tendons (implications for athletic activities). Muscles Ligaments Tendons J. 2014;4(3):298–302.
Frey C, Zamora J. The effects of obesity on orthopaedic foot and ankle pathology. Foot Ankle Int. 2007;28(9):996–9.
Abate M, Schiavone C, Di Carlo L, Salini V. Achilles tendon and plantar fascia in recently diagnosed type II diabetes: role of body mass index. Clin Rheumatol. 2012;31(7):1109–13.
Malliaras P, Cook JL, Kent PM. Anthropometric risk factors for patellar tendon injury among volleyball players. Br J Sports Med. 2007;41(4):259–63.
Klein EE, Weil L, Weil LS, Fleischer AE. Body mass index and Achilles tendonitis: a 10-year retrospective analysis. Foot Ankle Spec. 2013;6(4):276–82.
Kornaat PR, Reeder SB, Koo S, Brittain JH, Yu H, Andriacchi TP, et al. MR imaging of articular cartilage at 1.5T and 3.0T: comparison of SPGR and SSFP sequences. Osteoarthr Cartil. 2005;13:338–44.
Stahl R, Luke A, Ma CB, Krug R, Steinbach L, Majumdar S, et al. Prevalence of pathologic findings in asymptomatic knees of marathon runners before and after a competition in comparison with physically active subjects - a 3.0 T magnetic resonance imaging study. Skelet Radiol. 2008;37:627–38.
Calixto NE, Kumar D, Subburaj K, Singh J, Schooler J, Nardo L, et al. Zonal differences in meniscus MR relaxation times in response to in vivo static loading in knee osteoarthritis. J Orthop Res. 2016;34(2):249–61.
Culvenor AG, Collins NJ, Guermazi A, Cook JL, Vicenzino B, Khan KM, et al. Early knee osteoarthritis is evident one year following anterior cruciate ligament reconstruction: a magnetic resonance imaging evaluation. Arthritis Rheum. 2015;67(4):946–55.
Fleming BC, Fadale PD, Hulstyn MJ, Shalvoy RM, Oksendahl HL, Badger GJ, et al. The effect of initial graft tension after anterior cruciate ligament reconstruction: a randomized clinical trial with 36-month follow-up. Am J Sports Med. 2013;41(1):25–34.
Kaukinen P, Podlipská J, Guermazi A, Niinimäki J, Lehenkari P, Roemer FW, et al. Associations between MRI-defined structural pathology and generalized and localized knee pain – the Oulu Knee Osteoarthritis study. Osteoarthr Cartil. 2016;24(9):1565–76.
Kumar D, Subburaj K, Lin W, Karampinos DC, McCulloch CE, Li X, et al. Quadriceps and hamstrings morphology is related to walking mechanics and knee cartilage MRI relaxation times in young adults. J Orthop Sport Phys Ther. 2013;43(12):881–90.
Pan J, Pialat J-B, Joseph T, Kuo D, Joseph GB, Nevitt MC, et al. Knee cartilage T2 characteristics and evolution in relation to morphologic abnormalities detected at 3-T MR imaging: a longitudinal study of the normal control cohort from the Osteoarthritis Initiative. Radiology. 2011;261(2):507–15.
Sowers MF, Karvonen-Gutierrez CA, Jacobson JA, Jiang Y, Yosef M. Associations of anatomical measures from MRI with radiographically defined knee osteoarthritis score, pain, and physical functioning. J Bone Jt Surg Ser A. 2011;93(3):241–51.
Sritanyaratana N, Samsonov A, Mossahebi P, Wilson JJ, Block WF, Kijowski R. Cross-relaxation imaging of human patellar cartilage in vivo at 3.0T. Osteoarthr Cartil. 2014;22(10):1568–76.
Su F, Hilton JF, Nardo L, Wu S, Liang F, Link TM, et al. Cartilage morphology and T1ρ and T2 quantification in ACL-reconstructed knees: a 2-year follow-up. Osteoarthr Cartil. 2013;21(8):1058–67.
Van Der Heijden RA, De Kanter JLM, Bierma-Zeinstra SMA, Verhaar JAN, Van Veldhoven PLJ, Krestin GP, et al. Structural abnormalities on magnetic resonance imaging in patients with patellofemoral pain: a cross-sectional case-control study. Am J Sports Med. 2016;44(9):2339–46.
Thorlund JB, Juhl CB, Roos EM, Lohmander LS. Arthroscopic surgery for degenerative knee: systematic review and meta-analysis of benefits and harms. BMJ. 2015;350:h2747.
Sihvonen R, Paavola M, Malmivaara A, Itälä A, Joukainen A, Nurmi H, et al. Arthroscopic partial meniscectomy versus placebo surgery for a degenerative meniscus tear: a 2-year follow-up of the randomised controlled trial. Ann Rheum Dis. 2018;77(2):188–95.
Lanzer WL, Komenda G. Changes in articular cartilage after meniscectomy. Clin Orthop Relat Res. 1990;(252):41–8.
Song Y, Greve JM, Carter DR, Giori NJ. Meniscectomy alters the dynamic deformational behavior and cumulative strain of tibial articular cartilage in knee joints subjected to cyclic loads. Osteoarthr Cartil. 2008;16(12):1545–54.
Belo JN, Berger MY, Reijman M, Koes BW, Bierma-Zeinstra SMA. Prognostic factors of progression of osteoarthritis of the knee: a systematic review of observational studies. Arthritis Care Res. 2007;57(1):13–26.
Siddiqui MA, Ahmad I, Sabir AB, Ullah E, Rizvi SA, Rizvi SW. Clinical examination vs. MRI: evaluation of diagnostic accuracy in detecting ACL and meniscal injuries in comparison to arthroscopy. Polish Orthop Traumatol. 2013;78:59–63.
Brealey SD. Influence of magnetic resonance imaging of the knee on GPs’ decisions: a randomised trial. Br J Gen Pract. 2007;57(541):622–9.
Acknowledgements
We thank the investigators and institutions: University College London, the Royal National Orthopaedic Hospital, St George’s University Hospitals, Barts Health NHS Trust and the London Implant Retrieval Centre, for their involvement in the research.
Funding
This research study was funded by patient donations and supported by researchers at the National Institute for Health Research University College London Hospitals Biomedical Research Centre. The authors are also grateful to The Maurice Hatter Foundation, the RNOH Charity and the Rosetrees Trust for their support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
The study was approved by NHS Research Ethics Committee (REC Reference Number 15/LO/0086). All participants gave informed consent before taking part.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Laura M. Horga and Anna C. Hirschmann—joint first authorship
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Horga, L.M., Hirschmann, A.C., Henckel, J. et al. Prevalence of abnormal findings in 230 knees of asymptomatic adults using 3.0 T MRI. Skeletal Radiol 49, 1099–1107 (2020). https://doi.org/10.1007/s00256-020-03394-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-020-03394-z