Abstract
Background
Chloral hydrate is a sedative that has been used for magnetic resonance imaging (MRI).
Objective
To evaluate the use, effectiveness and safety of chloral hydrate administered by radiologists for the sedation of children who require MRI procedures.
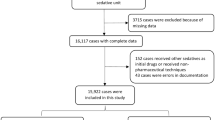
Materials and methods
We retrospectively reviewed the clinical charts for all patients ages 0 – 10 years old who underwent sedation with chloral hydrate for MRI from January 2000 to December 2010. Demographic factors, dose information, indication for MRI, therapeutic failures and adverse reactions to the drug were reviewed.
Results
One thousand, seven hundred and three children (946 males, 757 females) with a median age of 2.5 years (range: 4 days – 9.91 years) received chloral hydrate. Moderate to deep sedation was achieved in 1,618/1,703 (95%) of the patients, 35/1,703 (2.1%) of the patients failed to achieve moderate to deep sedation, and 47/1,703 (2.8%) of the patients woke up during MRI examination. Adverse reactions were present in 31/1,703 (1.8%) of the patients. Three severe adverse reactions occurred (0.18%). A single dose of chloral hydrate (40–60 mg/kg) was administered to 1,477/1,703 patients (86.7%). An additional dose of chloral hydrate (10–20 mg/kg), given 15 min after the first dose or when the patient woke up during the MRI examination, was required in 226/1,703 patients (13.3%). The likelihood of requiring an additional dose in children older than 2 years was 2.2 times the likelihood compared to children younger than 2 years (OR = 2.2 [95%CI: 1.6–3.0]). The use of a reduced dose (<50 mg/kg) was not associated with a higher therapeutic failure rate (OR = 1.04 [95%CI 0.57–1.89]).
Conclusion
Chloral hydrate is an appropriate sedation option for pediatric patients in MRI services when strict patient selection criteria are met. The use of a reduced dose does not affect the effectiveness of sedation. The lack of data regarding the presence of transient oxygen desaturation, the time to induce sedation and the exact duration of sedation are limitations of this study.
Similar content being viewed by others

References
Malviya S, Voepel-Lewis T, Eldevik OP et al (2000) Sedation and general anaesthesia in children undergoing MRI and CT: adverse events and outcomes. Br J Anaesth 84:743–748
Krauss B, Green SM (2006) Procedural sedation and analgesia in children. Lancet 367:766–780
Bracken J, Heaslip I, Ryan S (2012) Chloral hydrate sedation in radiology: retrospective audit of reduced dose. Pediatr Radiol 42:349–354
American Society of Anesthesiologists Task Force on Sedation and Analgesia by Non-Anesthesiologists (2002) Practice guidelines for sedation and analgesia by non-anesthesiologists. Anesthesiology 96:1004–1017
Olsen JW, Barger RL Jr, Doshi SK (2013) Moderate sedation: what radiologists need to know. AJR Am J Roentgenol 201:941–946
Williams K, Thomson D, Seto I et al (2012) Standard 6: age groups for pediatric trials. Pediatrics 129:S153–S160
Lee YJ, Kim do K, Kwak YH et al (2012) Analysis of the appropriate age and weight for pediatric patient sedation for magnetic resonance imaging. Am J Emerg Med 30:1189–1195
Cortellazzi P, Lamperti M, Minati L et al (2007) Sedation of neurologically impaired children undergoing MRI: a sequential approach. Paediatr Anaesth 17:630–636
Cutter TW (2009) Radiologists and anesthesiologists. Anesthesiol Clin 27:95–106
Daniel FB, DeAngelo AB, Stober JA et al (1992) Hepatocarcinogenicity of chloral hydrate, 2-chloroacetaldehyde, and dichloroacetic acid in the male B6C3F1 mouse. Fundam Appl Toxicol 19:159–168
American Academy of Pediatrics Committee on Drugs and Committee on Environmental Health (1993) Use of chloral hydrate for sedation in children. Pediatrics 92:471–473
Litman RS, Soin K, Salam A (2010) Chloral hydrate sedation in term and preterm infants: an analysis of efficacy and complications. Anesth Analg 110:739–746
U.S. Food and Drug Administration (2013) Drugs to be discontinued. http://www.fda.gov/drugs/drugsafety/drugshortages/ucm050794.htm. Accessed 4 January 2014
Matich SM (2012) Just pediatrics: a eulogy for chloral hydrate. J Radiol Nur 31:152–153
Greenberg SB, Faerber EN, Aspinall CL et al (1993) High-dose chloral hydrate sedation for children undergoing MR imaging: safety and efficacy in relation to age. AJR Am J Roentgenol 161:639–641
Sury MR, Hatch DJ, Deeley T et al (1999) Development of a nurse-led sedation service for paediatric magnetic resonance imaging. Lancet 353:1667–1671
Low E, O’Driscoll M, MacEneaney P et al (2008) Sedation with oral chloral hydrate in children undergoing MRI scanning. Ir Med J 101:80–82
McCarver-May DG, Kang J, Aouthmany M et al (1996) Comparison of chloral hydrate and midazolam for sedation of neonates for neuroimaging studies. J Pediatr 128:573–576
Beauve B, Dearlove O (2008) Sedation of children under 4 weeks of age for MRI examination. Paediatr Anaesth 18:892–893
Schmerler BL, Cohen DM, Leder MS et al (2008) Procedural sedation for fracture reduction in children with hyperactivity. Am J Emerg Med 26:661–664
Acknowledgments
We want to thank all the nurses in our department who, with their professionalism and hard work, provided a safe and comfortable environment for the sedation to the children.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Delgado, J., Toro, R., Rascovsky, S. et al. Chloral hydrate in pediatric magnetic resonance imaging: evaluation of a 10-year sedation experience administered by radiologists. Pediatr Radiol 45, 108–114 (2015). https://doi.org/10.1007/s00247-014-3091-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-014-3091-0