Abstract
Background
Chloral hydrate (CH) is safe and effective for sedation of suitable children.
Objective
The purpose of this study was to assess whether adequate sedation is achieved with reduced CH doses.
Materials and methods
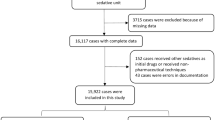
We retrospectively recorded outpatient CH sedations over 1 year. We defined standard doses of CH as 50 mg/kg (infants) and 75 mg/kg (children >1 year). A reduced dose was defined as at least 20% lower than the standard dose.
Results
In total, 653 children received CH sedation (age, 1 month–3 years 10 months), 42% were given a reduced initial dose. Augmentation dose was required in 10.9% of all children, and in a higher proportion of children >1 year (15.7%) compared to infants (5.7%; P < 0.001). Sedation was successful in 96.7%, and more frequently successful in infants (98.3%) than children >1 year (95.3%; P = 0.03). A reduced initial dose had no negative effect on outcome (P = 0.19) or time to sedation. No significant complications were seen.
Conclusion
We advocate sedation with reduced CH doses (40 mg/kg for infants; 60 mg/kg for children >1 year of age) for outpatient imaging procedures when the child is judged to be quiet or sleepy on arrival.
Similar content being viewed by others
References
American Academy of Pediatrics Committee on Drugs (1992) American Academy of Pediatrics Committee on Drugs: Guidelines for monitoring and management of pediatric patients during and after sedation for diagnostic and therapeutic procedures. Pediatrics 89:1110–1115
Committee on Drugs. American Academy of Pediatrics (2002) Guidelines for monitoring and management of pediatric patients during and after sedation for diagnostic and therapeutic procedures: addendum. Pediatrics 110:836–838
Vade A, Sukhani R, Dolenga M et al (1995) Chloral hydrate sedation of children undergoing CT and MR imaging: safety as judged by American Academy of Pediatrics guidelines. AJR 165:905–909
Sanborn PA, Michna E, Zurakowski D et al (2005) Adverse cardiovascular and respiratory events during sedation of pediatric patients for imaging examinations. Radiology 237:288–294
Egelhoff JC, Ball WS Jr, Koch BL et al (1997) Safety and efficacy of sedation in children using a structured sedation program. AJR 168:1259–1262
Greenberg SB, Faerber EN, Aspinall CL et al (1993) High-dose chloral hydrate sedation for children undergoing MR imaging: safety and efficacy in relation to age. AJR 161:639–641
American Academy of Pediatrics Committee on Drugs and Committee on Environmental Health (1993) American Academy of Pediatrics Committee on Drugs and Committee on Environmental Health: Use of chloral hydrate for sedation in children. Pediatrics 92:471–473
Haselkorn T, Whittemore AS, Udaltsova N et al (2006) Short-term chloral hydrate administration and cancer in humans. Drug Safety 29:67–77
Sury M, Bullock I, Rabar S et al (2010) Sedation for diagnostic and therapeutic procedures in children and young people: summary of NICE guidance. BMJ 341:c6819
Dearlove O, Corcoran JP (2007) Sedation of children undergoing magnetic resonance imaging. Br J Anaesth 98:548–549
Fogel MA, Weinberg PM, Parave E et al (2008) Deep sedation for cardiac magnetic resonance imaging: a comparison with cardiac anesthesia. J Pediatr 152:534–539, e531
Low E, O’Driscoll M, MacEneaney P et al (2008) Sedation with oral chloral hydrate in children undergoing MRI scanning. Ir Med J 101:80–82
Malviya S, Voepel-Lewis T, Eldevik OP et al (2000) Sedation and general anaesthesia in children undergoing MRI and CT: adverse events and outcomes. Br J Anaesth 84:743–748
Malviya S, Voepel-Lewis T, Prochaska G et al (2000) Prolonged recovery and delayed side effects of sedation for diagnostic imaging studies in children. Pediatrics 105:E42
Ong HT, Lim KJ, Low PC et al (2004) Simple instructions for partial sleep deprivation prior to pediatric EEG reduces the need for sedation. Clin Neurophysiol 115:951–955
Shields CH, Johnson S, Knoll J et al (2004) Sleep deprivation for pediatric sedated procedures: not worth the effort. Pediatrics 113:1204–1208
Sury MR, Hatch DJ, Deeley T et al (1999) Development of a nurse-led sedation service for paediatric magnetic resonance imaging. Lancet 353:1667–1671
Acknowledgements
The authors wish to acknowledge the role of the radiology nurses who are principally responsible for the success of sedation in our department.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bracken, J., Heaslip, I. & Ryan, S. Chloral hydrate sedation in radiology: retrospective audit of reduced dose. Pediatr Radiol 42, 349–354 (2012). https://doi.org/10.1007/s00247-011-2279-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-011-2279-9