Abstract
Purpose
The ability to predict and detect clinical and subclinical nephrotoxicity early in the course of therapy has the potential to improve long-term outcomes in cancer patients receiving cisplatin chemotherapy. Pharmacokinetic parameters could serve as predictors of cisplatin-induced nephrotoxicity.
Methods
Participants [n = 13] were treated with a 1-h cisplatin infusion [30–75 mg/m2]. Blood was collected pre-dose and up to 6 h post-dose. Urinary biomarkers [KIM-1, calbindin, clusterin, GST-pi, β2M, albumin, NGAL, osteopontin, clusterin, MCP-1, cystatin C, and TFF3] were measured at baseline, days 3 and 10. Total and unbound platinum concentrations were measured using ICP/MS. Noncompartmental analysis was performed, and correlation and regression analyses evaluated the relationships between platinum pharmacokinetics and nephrotoxicity.
Results
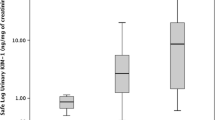
Peak platinum urinary concentrations correlated with urinary levels of KIM-1, calbindin, clusterin, GST-pi, β2M, albumin, NGAL, osteopontin, clusterin, cystatin C, and TFF3 at day 10. Unbound platinum plasma concentrations at 2 h also correlated with urinary clusterin, β2M, cystatin C, NGAL, osteopontin, and TFF3 at day 3. Regression analyses suggested 2-h total plasma platinum concentrations greater than 2000 ng/ml, and peak urinary platinum concentrations above 24,000 ng/ml may serve as potential approximations for elevated risk of nephrotoxicity. Platinum area under the plasma concentration time curve was associated with serum creatinine and estimated glomerular filtration rate.
Conclusions
Peak plasma and urinary platinum concentrations and pharmacokinetic parameters were associated with risk of subclinical cisplatin-induced kidney injury as assessed using novel urinary biomarkers. Future studies will examine these relationships in larger clinical populations of cisplatin-induced acute kidney injury.

Similar content being viewed by others
References
Pabla N, Dong Z (2008) Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int 73(9):994–1007
Arany I, Safirstein RL (2003) Cisplatin nephrotoxicity. Semin Nephrol 23(5):460–464
Khwaja A (2012) KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 120(4):c179–c184
Devarajan P (2010) The use of targeted biomarkers for chronic kidney disease. Adv Chronic Kidney Dis 17(6):469–479
Dieterle F, Sistare F, Goodsaid F, Papaluca M, Ozer JS, Webb CP, Baer W, Senagore A, Schipper MJ, Vonderscher J, Sultana S, Gerhold DL, Phillips JA, Maurer G, Carl K, Laurie D, Harpur E, Sonee M, Ennulat D, Holder D, Andrews-Cleavenger D, Gu YZ, Thompson KL, Goering PL, Vidal JM, Abadie E, Maciulaitis R, Jacobson-Kram D, Defelice AF, Hausner EA, Blank M, Thompson A, Harlow P, Throckmorton D, Xiao S, Xu N, Taylor W, Vamvakas S, Flamion B, Lima BS, Kasper P, Pasanen M, Prasad K, Troth S, Bounous D, Robinson-Gravatt D, Betton G, Davis MA, Akunda J, McDuffie JE, Suter L, Obert L, Guffroy M, Pinches M, Jayadev S, Blomme EA, Beushausen SA, Barlow VG, Collins N, Waring J, Honor D, Snook S, Lee J, Rossi P, Walker E, Mattes W (2010) Renal biomarker qualification submission: a dialog between the FDA-EMEA and predictive safety testing consortium. Nat Biotechnol 28(5):455–462
Shinke H, Masuda S, Togashi Y, Ikemi Y, Ozawa A, Sato T, Kim YH, Mishima M, Ichimura T, Bonventre JV, Matsubara K (2015) Urinary kidney injury molecule-1 and monocyte chemotactic protein-1 are noninvasive biomarkers of cisplatin-induced nephrotoxicity in lung cancer patients. Cancer Chemother Pharmacol 76(5):989–996
Vaidya VS, Ozer JS, Dieterle F, Collings FB, Ramirez V, Troth S, Muniappa N, Thudium D, Gerhold D, Holder DJ, Bobadilla NA, Marrer E, Perentes E, Cordier A, Vonderscher J, Maurer G, Goering PL, Sistare FD, Bonventre JV (2010) Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nat Biotechnol 28(5):478–485
Pinches M, Betts C, Bickerton S, Burdett L, Thomas H, Derbyshire N, Jones HB, Moores M (2012) Evaluation of novel renal biomarkers with a cisplatin model of kidney injury: gender and dosage differences. Toxicol Pathol 40(3):522–533
George B et al (2016) Profiling of kidney injury biomarkers in patients receiving cisplatin: time-dependent changes in the absence of clinical nephrotoxicity. Clin Pharmacol Ther 101(4):510–518
Erdlenbruch B, Nier M, Kern W, Hiddemann W, Pekrun A, Lakomek M (2001) Pharmacokinetics of cisplatin and relation to nephrotoxicity in paediatric patients. Eur J Clin Pharmacol 57(5):393–402
Nagai N, Ogata H (1997) Quantitative relationship between pharmacokinetics of unchanged cisplatin and nephrotoxicity in rats: importance of area under the concentration-time curve (AUC) as the major toxicodynamic determinant in vivo. Cancer Chemother Pharmacol 40(1):11–18
Nagai N, Kinoshita M, Ogata H, Tsujino D, Wada Y, Someya K, Ohno T, Masuhara K, Tanaka Y, Kato K, Nagai H, Yokoyama A, Kurita Y (1996) Relationship between pharmacokinetics of unchanged cisplatin and nephrotoxicity after intravenous infusions of cisplatin to cancer patients. Cancer Chemother Pharmacol 39(1–2):131–137
Skali H, Uno H, Levey AS, Inker LA, Pfeffer MA, Solomon SD (2011) Prognostic assessment of estimated glomerular filtration rate by the new chronic kidney disease epidemiology collaboration equation in comparison with the modification of diet in renal disease study equation. Am Heart J 162(3):548–554
Ma J, Stoter G, Verweij J, Schellens JHM (1996) Comparison of ethanol plasma-protein precipitation with plasma ultrafiltration and trichloroacetic acid protein precipitation for the measurement of unbound platinum concentrations. Cancer Chemother Pharmacol 38(4):391–394
Xie R, Johnson W, Rodriguez L, Gounder M, Hall GS, Buckley B (2007) A study of the interactions between carboplatin and blood plasma proteins using size exclusion chromatography coupled to inductively coupled plasma mass spectrometry. Anal Bioanal Chem 387(8):2815–2822
O’brien RM (2007) A caution regarding rules of thumb for variance inflation factors. Qual Quant 41(5):673–690
van Warmerdam LJ et al (1995) The use of the Calvert formula to determine the optimal carboplatin dosage. J Cancer Res Clin Oncol 121(8):478–486
Haase M, Kellum JA, Ronco C (2012) Subclinical AKI--an emerging syndrome with important consequences. Nat Rev Nephrol 8(12):735–739
Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fielitz A, NGAL Meta-analysis Investigator Group (2009) Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis 54(6):1012–1024
Yasumasu T, Ueda T, Uozumi J, Mihara Y, Kumazawa J (1992) Comparative study of cisplatin and carboplatin on pharmacokinetics, nephrotoxicity and effect on renal nuclear DNA synthesis in rats. Pharmacol Toxicol 70(2):143–147
Fukushima K, Okada A, Sasaki K, Kishimoto S, Fukushima S, Hamori M, Nishimura A, Shibata N, Shirai T, Terauchi R, Kubo T, Sugioka N (2016) Population pharmacokinetic-toxicodynamic modeling and simulation of cisplatin-induced acute renal injury in rats: effect of dosing rate on nephrotoxicity. J Pharm Sci 105(1):324–332
Madias NE, Harrington JT (1978) Platinum nephrotoxicity. Am J Med 65(2):307–314
Acknowledgements
The authors wish to thank the participants in this study.
Funding
Grant funding support for this study was provided by National Institutes of Health funding numbers T32ES007148, R21DK093903, P30ES005022, and P30CA072720.
Author information
Authors and Affiliations
Contributions
MI analyzed data and wrote the manuscript. CC, NM, MG, CLO, and DWB contributed to conduct of the patient study. YH and SLH contributed to the analysis plan. BG, XW, and BB performed biomarker assays and/or platinum analyses. MSJ and LA conceived, designed and conducted the study, and oversaw all components of the analytical and manuscript plan. All authors contributed to editing the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Electronic supplementary material
ESM 1
(DOCX 19 kb)
Rights and permissions
About this article
Cite this article
Ibrahim, M.E., Chang, C., Hu, Y. et al. Pharmacokinetic determinants of cisplatin-induced subclinical kidney injury in oncology patients. Eur J Clin Pharmacol 75, 51–57 (2019). https://doi.org/10.1007/s00228-018-2552-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-018-2552-z