Abstract
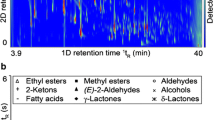
A variety of extraction methodologies were applied to chinotto (Citrus myrtifolia) fruits from Sicily, along with sensory and chemical analyses. By gas chromatographic techniques, either in monodimensional (GC-FID, GC–MS) or in multidimensional (MDGC) fashion, it was established how the isolation procedure affected the volatile fingerprint of such fruit. In general, limonene, linalyl acetate, myrcene, β-pinene, α-pinene, (E)-β-ocimene, linalool and geranyl acetate resulted to be the predominant volatiles. However, although revealed at lower levels, other compounds, such as trans-linalool oxide, perilla alcohol, trans-limonene oxide, may be responsible for peculiar olfactory notes. Compounds such as linalool, myrcene, β-pinene, octanal, decanal, and geranyl and perillyl acetates were selectively extracted by blending plus the addition of solvents. (E)-β-Ocimene and nootkatone were considerably expressed in hand-squeezed and solvent-extracted samples, respectively. On the other hand, linalyl acetate was the most abundant compound in samples extracted by solvent. Concerning the sensory evaluations, odor characters varied depending on the oil extraction methodology. Indeed, the flowery and citrus notes were perceived in all samples by the majority of panelists; conversely, the minty attribute was the one least smelled in five out of six samples. Enantio-MDGC analysis highlighted seven chiral pairs, with the following enantiomeric ratios: (−)/(+) limonene (1.8/98.2), (−)/(+) linalyl acetate (99.4/0.6), (+)/(−) β-pinene (99.8/0.2), (−)/(+) linalool (5.5/94.5), (+)/(−) terpinen-4-ol (48.9/51.1), (−)/(+) α-pinene (22.3/77.7) and (−)/(+) α-terpineol (20.5/79.5).



Similar content being viewed by others
References
Scordino M, Sabatino L, Belligno A, Gagliano G (2011) Flavonoids and furocoumarins distribution of unripe chinotto (Citrus × myrtifolia Rafinesque) fruit: beverage processing homogenate and juice characterization. Eur Food Res Technol 233:759–767
Webber HJ, Batchelor LD (1943) In: University of California Press (ed) The citrus industry. vol 1. History, botany and breeding. University of California Press, Berkeley
Chinotto: cronache da un altro bere (2018) http://chinotto.cpenti.it/2015. Accessed 20 July 2018
Cautela D, Pirrello AG, Esposito C, Minasi P (2004) Composition of chinotto (Citrus myrtifolia): part I. Essenze Deriv Agrum 74:49–55
Cautela D, Pirrello AG, Esposito C, Siano F, Castaldo D (2004) Determination of trace levels of heavy metals in citrus juices: chinotto and red orange. Essenze Deriv Agrum 74:11–17
Barreca D, Bellocco E, Caristi C, Leuzzi U, Gattuso G (2010) Flavonoid composition and antioxidant activity of juices from chinotto (Citrus × myrtifolia Raf.) fruits at different ripening stages. J Agric Food Chem 58:3031–3036
Scordino M, Sabatino L, Belligno A, Gagliano G (2011) Characterization of polyphenolic compounds in unripe chinotto (Citrus × myrtifolia) fruit by HPLC/PDA/ESI/MS-MS. Nat Prod Commun 6:1857–1862
Barreca D, Bellocco E, Caristi C, Leuzzi U, Gattuso G (2011) Elucidation of the flavonoid and furocoumarin composition and radical-scavenging activity of green and ripe chinotto (Citrus × myrtifolia Raf.) fruit tissues, leaves and seeds. Food Chem 129:1504–1512
Protti M, Valle F, Poli F, Raggi MA, Mercolini L (2015) Bioactive molecules as authenticity markers of Italian chinotto (Citrus × myrtifolia) fruits and beverages. J Pharm Biomed Anal 104:75–80
Costa R, Salvo A, Rotondo A, Bartolomeo G, Pellizzeri V, Saija E, Arrigo S, Interdonato M, Trozzi A, Dugo G (2018) Combination of separation and spectroscopic analytical techniques: application to compositional analysis of a minor citrus species. Nat Prod Res. https://doi.org/10.1080/14786419.2018.1428597
Chialva F, Doglia G (1990) Essential oil constituents of chinotto (Citrus aurantium L. var. myrtifolia Guill.). J Essent Oil Res 2:33–35
Verzera A, Stagno d’Alcontres I, Trozzi A, Saitta M (1991) On the genuineness of citrus essential oils. Part XXXVIII. The composition of the volatile fraction of chinotto essential oil. Essenze Deriv Agrum 61:323–330
Salvo A, Bruno M, La Torre GL, Vadalà R, Mottese AF, Saija E, Mangano V, Casale KE, Cicero N, Dugo G (2016) Interdonato lemon from Nizza di Sicilia (Italy): chemical composition of hexane extract of lemon peel and histochemical investigation. Nat Prod Res 30:1517–1525
Adams RP (2007) In: Allured Publishing Corporation (ed) Identification of essential oil components by gas chromatography/mass spectrometry, 4th edn. Allured Publishing Corporation, Carol Stream
Mondello L (2011) FFNSC 2: Flavors and fragrances of natural and synthetic compounds, mass spectral database. Wiley, Hoboken
Nist WebBook (2018) National Institute of Standard and Technology. https://webbook.nist.gov/chemistry. Accessed 10 July 2018
Deterre S, Rega B, Delarue J, Decloux M, Lebrun M, Giampaoli P (2012) Identification of key aroma compounds from bitter orange (Citrus aurantium L.) products: essential oil and macerate–distillate extract. Flavour Fragr J 27:77–88
Mastello RB, Capobiango M, Chin ST, Monteiro M, Marriott PJ (2015) Identification of odour-active compounds of pasteurised orange juice using multidimensional gas chromatography techniques. Food Res Int 75:281–288
Minh Tu NT, Onishi Y, Choi HS, Kondo Y, Bassore SM, Ukeda H, Sawamura M (2002) Characteristic odor components of Citrus sphaerocarpa Tanaka (Kabosu) cold-pressed peel oil. J Agric Food Chem 50:2908–2913
Costa R, Albergamo A, Bua GD, Saija E, Dugo G (2017) Determination of flavor constituents in particular types of flour and derived pasta by heart-cutting multidimensional gas chromatography coupled with mass spectrometry and multiple headspace solid-phase microextraction. LWT Food Sci Technol 86:99–107
Bua GD, Albergamo A, Annuario G, Zammuto V, Costa R, Dugo G (2017) High-throughput ICP-MS and chemometrics for exploring the major and trace element profile of the Mediterranean sepia ink. Food Anal Methods 10:1181–1190
Albergamo A, Mottese AF, Bua GD, Caridi F, Sabatino G, Barrega L, Costa R, Dugo G (2018) Discrimination of the Sicilian prickly pear (Opuntia ficus-indica L., CV. Muscaredda) according to the provenance by testing unsupervised and supervised chemometrics. J Food Sci. https://doi.org/10.1111/1750-3841.14382
Albergamo A, Bua GD, Rotondo A, Bartolomeo G, Annuario G, Costa R, Dugo G (2018) Transfer of major and trace elements along the “farm-to-fork” chain of different whole grain products. J Food Comp Anal 66:212–220
Mottese AF, Albergamo A, Bartolomeo G, Bua GD, Rando R, De Pasquale P, Saija E, Donato D, Dugo G (2018) Evaluation of fatty acids and inorganic elements by multivariate statistics for the traceability of the Sicilian Capparis spinosa L. J Food Comp Anal 72:64–66
Murase T, Misawa K, Haramisu S, Minegishi Y, Hase T (2010) Nootkatone, a characteristic constituent of grapefruit, stimulates energy metabolism and prevents diet-induced obesity by activating AMPK. Am J Physiol Endocrinol Metab 299:E266–E275
Haro-Guzmán L (2011) In: CRC Press (ed) Citrus oils—composition, advanced analytical techniques, contaminants, and biological activity. CRC Press, Boca Raton
Guenter Berger R (2007) Berger RG (ed) Flavours and fragrances. Chemistry, bioprocessing and sustainability. Springer, Berlin Heidelberg
Choi HS (2003) Character impact odorants of Citrus Hallabong [(C. unshiu Marcov × C. sinensis Osbeck) × C. reticulata Blanco] cold-pressed peel oil. J Agric Food Chem 51:2687–2692
Sciarrone D, Costa R, Ragonese C, Tranchida PQ, Santi L, Dugo P, Dugo G, Mondello L (2011) Application of a multidimensional gc system with simultaneous mass spectrometric and FID detection to the analysis of sandalwood oil. J Chromatogr A 1218:137–142
Dugo G, Mondello L (2011) In: CRC Press (ed) Citrus oils—composition, advanced analytical techniques, contaminants, and biological activity. CRC Press, Boca Raton
Lo Presti M, Sciarrone D, Crupi ML, Costa R, Ragusa S, Dugo G, Mondello L (2008) Evaluation of the volatile and chiral composition in Pistacia lentiscus L. essential oil. Flavour Fragr J 23:249–257
Costa R, Zellner B, Crupi ML, De Fina MR, Valentino MR, Dugo P, Dugo G, Mondello L (2008) Gas chromatography-mass spectrometry (GC–MS), gas chromatography-olfactometry (GC-O) and enantio-GC investigation on the essential oil of Tarchonanthus camphoratus L. Flavour Fragr J 23:40–48
Liberto E, Cagliero C, Sgorbini B, Bicchi C, Sciarrone D, D’Acampora Zellner B, Mondello L, Rubiolo P (2008) Enantiomer identification in the flavour and fragrance field by “interactive” combination of linear retention indices from enantioselective gas chromatography and mass spectrometry. J Chromatogr A 1195:117–126
Di Bella G, Naccari C, Bua GD, Rastrelli L, Lo Turco V, Potortì AG, Dugo G (2016) Mineral composition of some varieties of beans from mediterranean and tropical areas. Int J Food Sci Nutr 67:239–248
Potortì AG, Lo Turco V, Saitta M, Bua GD, Tropea A, Dugo G, Di Bella G (2017) Chemometric analysis of minerals and trace elements in Sicilian wines from two different grape cultivars. Nat Prod Res 31:1000–1005
Potortì AG, Di Bella G, Mottese AF, Bua GD, Fede MR, Sabatino G, Salvo A, Somma R, Dugo G, Lo Turco V (2018) Traceability of Protected Geographical Indication (PGI) Interdonato lemon pulps by chemometric analysis of the mineral composition. J Food Compos Anal 69:122–128
Albergamo A, Rotondo A, Salvo A, Pellizzeri V, Bua GD, Maggio A, Dugo G (2017) Metabolite and mineral profiling of “Violetto di Niscemi” and “Spinoso di Menfi” globe artichokes by 1H-NMR and ICP-MS. Nat Prod Res 31:990–999
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Salvo, A., Costa, R., Albergamo, A. et al. An in-depth study of the volatile variability of chinotto (Citrus myrtifolia Raf.) induced by the extraction procedure. Eur Food Res Technol 245, 873–883 (2019). https://doi.org/10.1007/s00217-019-03232-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-019-03232-0