Abstract
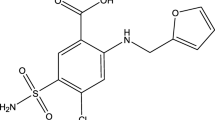
The activity of the α-l-fucosidase (AFU) enzyme represents an excellent test for diagnosis of hepatocellular carcinoma (HCC) and fucosidosis recognized in inborn disorder of metabolism and increases the sensitivity of detection to 95.5% in patients with HCC. Therefore, the determination of the activity of AFU enzyme is very important and can be used as a screening tool for the early diagnosis of tumors for HCC patients. A simple, accurate, and sensitive potentiometric method was developed for measuring the activity of AFU. The method was based upon measuring the concentration of 2-chloro-4-nitrophenol (2-chloro-4-NP) using a 2-chloro-4-NP-rhodamine B ion pair in a PVC membrane sensor. The electrode shows a linear, reproducible, and stable potentiometric response with an anionic Nernstian slope of −51.13 ± 0.6 mV/decade over a wide range of concentrations 10−5–10−2 M and a detection limit of 1.0 × 10−6 M of 2-chloro-4-NP. The membrane exhibits a fast response time of 30 s, over a pH range of 4.0–6.5. The selectivity coefficients indicate excellent selectivity for 2-chloro-4-NP over a number of interfering species, e.g., chloride, nitrate, sulfate, chromate urea, albumin, glucose, uric acid, and total protein. The prepared sensor has been used successfully for the determination of 2-chloro-4-NP produced from the hydrolysis of 2-chloro-4-NP-α-l-fucopyranoside substrate. It was also applied for the determination α-l-fucosidase enzyme of 33 serum samples of healthy subjects and patients. The average recoveries ± RSD for the healthy subjects, cirrhosis of chronic hepatitis C and B, and HCC serum samples were 102.6 ± 1.01%, 101.5 ± 0.95%, and 100.1 ± 1.1%, respectively. The results obtained are in good agreement with those obtained by standard methods.

Potentiometric response of 2-chloro-4-NP using 2-chloro-4-NP-Rd PVC membrane sensor in phosphate buffer of pH 5 at 25 °C






Similar content being viewed by others
References
Bosch FX, Ribes J, Borras J (1999) Epidemiology of primary liver cancer. Semin Liver Dis 19:271–285
Bosch FX, Ribes J, Diaz M, Cleries R (2004) Primary liver cancer: worldwide incidence and trends. Gastroenterology 127:S5–S16
Liaw YF, Tai DI, Chu CM, Lin DY, Sheen IS, Chen TJ, Pao CC (1986) Early detection of hepatocellular carcinoma in patients with chronic type B hepatitis. A prospective study. Gastroenterology 90:263–267
Zhang BH, Yang BH, Tang ZY (2004) Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol 130:417–421
Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodes J (2001) Clinical management of hepatocellular carcinoma. J Hepatol 35:421–430
Van Hoof F, Hers HG (1968) The abnormalities of lysosomal enzymes in mucopolysacc haridoses. Eur J Biochem 7:34–44
Johnson SW, Alhadeff JA (1991) Mammalian a-fucosidase. CompBiochem Physiol 99B:479–488
Deugnier Y, David V, Brissot P, Mabo P, Delamaire D, Messner M et al (1984) Serum a-fucosidase: a new marker for the diagnosis of primary hepatic carcinoma. Hepatology 4(5):4889–4892
Deugnier Y, David V, Leray G, Blayau M, Le Gall JY (1988) Marquers biologiques du carcinome hepato-cellulaire. Ann Biol Clin (Paris) 46:85–88
Takashi H, Saibara T, Iwamure S et al (1994) α-l-fucosidase activity and tumor size in hepatocellular carcinoma. Hepatology 19:1414–1417
Boucau J, Sanki AK, Voss BJ, Sucheck SJ, Ronning DR (2009) A coupled assay measuring Mycobacterium tuberculosis antigen 85C enzymatic activity. Anal Biochem 385:120–127
Toyoda H, Kumada T, Kaneoka Y, Osaki Y, Kimura T, Arimoto A, Oka H, Yamazaki O, Manabe T, Chung H, Kudo M, Matsunaga T (2008) Prognostic value of pretreatment levels of tumor markers for hepatocellular carcinoma on survival after curative treatment of patients with HCC. J Hepatol 49:223–232
Oka H, Tamori A, Kuroki T, Kobayashi K, Yammamoto S (1994) Prospective study of alpha-fetoprotein in cirrhotic patients monitored for development of hepatocellular carcinoma. Hepatology 19:61–66
El-Houseini ME, Mohammed Mohammed S, Elshemey WM, Hussein DT, Desouky SO, Elsayed AA (2005) Enhanced detection of hepatocellular carcinoma. Cancer Control 12:248–253
Toyoda H, Kumada T, kaneoka Y, Osaki Y, Kimura T, Arimoto A, Oka H, Yamazaki O, Manabe T, Chung H, Kudo M (2008) Prognostic value of pretreatment levels of tumor markers for hepatocellular carcinoma on survival after curative treatment of patients with HCC. J Hepatol 49:223–232
El-Houseini ME, El-Sherbiny M, Awad ME et al (2001) Serum alpha-l-fucosidase enzyme activity as a marker for hepatocellular carcinoma: comparison with AFP using ROC analysis. J Egypt Nat Cancer Inst 4:277–283
Shu HJ, Saito T, Watanabe H (2002) Expression of the Musashi1 gene encoding the RNA-binding protein in human hepatoma cell lines. Biochem Biophys Res Commun 293:150–154
Nakatsura T, Yoshitake S, Senju S (2003) over expressed specifically in human hepatocellular carcinoma, is a novel tumor marker. Biochem Biophys Res Commun 306:16–25
Blumbeig S, Hildesheim J, Yariv J, Wilson KJ (1972) The use of I-amino-l-fucose bound to sepharose in the isolation of l-fucose-binding proteins. Biochim Biophys Acta 264:171–176
Lewy GA, McAllan A (1961) Mammalian fucosidases 2. α-l-Fucosidas. Biochem J 80:435–439
Van Hoof F, Hers HG (1968) Mucopolysaccharidoses by absence of α-fucosidase. Lancet 1:11193–1198
Deugnier Y, David V, Brissot P (1984) Serum α-l-fucosidase: a new marker for the diagnosis of primary hepatic carcinoma. J Hepatol 4:889–892
Johnson SW, Alhadeff JA (1991) Mammalian α-l-fucosidases. Comp Biochem Physiol B Biochem Mol Biol 99:479–488
Durand P, Borrone C, Della C, Fucosidosis (1969) J Pediatr 75:665–674
Zietke K, Okada S, Brien JSO (1972) Fucosidosis diagnosis by serum assay α-l-fucosidase. J Lab Clin Med 79:1649–1654
Troost J, Van der Heijden MC, Staal GE (1976) Characterization of alpha-l-fucosidase from two different families with fucosidosis. Clin Chim Acta 73:329–346
Ju-Jun W, En-Hua C (2004) Rapid kinetic rate assay of the serum α-l-fucosidase in patients with hepatocellular carcinoma by using a novel substrate. Clin Chim Acta 347:103–109
Kasai K, Okada K, Yamatsugu, N (1992) Pat Jan Appl 92353689. 15 December
Wood SA (1975) Sensitive fluorometric assay for α-l-fucosidase. Clin Chim Acta 58:251–256
Cuer M, Barnier A, de La Salmoniere P, Durand G, Seta N (2000) Fluorimetric measurement of plasma alpha-l-fucosidase activity with a centrifugal analyzer: reference values in a healthy French adult population. Clin Chem 46:560–576
El-Shahawi MS, Othman AM, El-Houseini ME, Nashed B, Elsofy MS (2009) Spectrofluorimetric method for measuring the activity of the enzyme-l-fucosidase using the ion associate of 2-chloro-4-nitro phenol–rhodamine-B. Talanta 80:19–23
Atteia MS, Othman AM, Aboaly M, Abdel Mottelab MS (2010) Novel spectrofluorimetric method for measuring the activity of the enzyme r-l-fucosidase using the nano composite optical sensor samarium(III)-doxycycline complex doped in sol-gel matrix. Anal Chem 82:6230–6236
Miura T, Okamoto K, Yanase H (2005) Purification and characterization of extracellular 1,2-α-l-fucosidase from Bacillus cereus. J Biosci Bioeng 99:629–635
Ho MW, Brien JO’ (1971) Gaucher’s disease: deficiency of “cid” β-glucosidase and reconstitution of enzyme activity in vitro. Proc Natl Acad Sci U S A 68:2810–2813
IUPAC (1995) Analytical chemistry division, commission on analytical nomenclature. Pure Appl Chem 67:507
Armstrong RD, Covington AK, Proud WG (1988) Solvent properties of PVC membranes. J Electroanal Chem 257:155–161
Tseng SJ, Hsu JP (1990) A comparison of the parameter estimating procedures for the Michaelis–Menten model. J Theor Biol 145(4):457–464
Bravo IG, Busto F, De Arriaga D et al (2001) A normalized plot as a novel and time-saving tool in complex enzyme kinetic analysis. Biochem J 358(Pt 3):573–583
Mortensson-Egnund K, Schbyen R, Howe C, Lee LT, Harboe J (1969) α-l-fucosidase from a soil bacterium. J Bacteriol 98:924–929
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Othman, A.M., El-Houseini, M.E., El-Sofy, M.S. et al. Potentiometric determination of α-l-fucosidase enzyme by using 2-chloro-4-nitrophenol-rhodamine B ion pair chemical recognition in PVC membrane sensor. Anal Bioanal Chem 400, 787–795 (2011). https://doi.org/10.1007/s00216-011-4774-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-011-4774-0