Abstract
Background
Fabrication of two membrane sensors using two acidic indicators among sulphonthalein dyes, namely bromophenol blue (BPB) and bromocresol green (BCG), and their use as indicative electrodes for the quantification of frusemide (FUR) is presented. The ion pair complexes of FUR with BPB or BCG are used to prepare the membranes in THF solvent, PVC matrix and dibutyl phthalate (DBP) as plasticizer and subsequently to fabricate FUR-BPB (Sensor I) and FUR-BCG (Sensor II) sensors.
Results
Sensors I and II are employable to determine 2.4 × 10-5–2.4 × 10-3 mol/L FUR at operative pH of 3.71. The calibration curve between the potentials against the concentration of FUR yielded the slopes of 58.73 ± 1 and 57.66 ± 1 mV/decade, respectively, using Sensors I and II, and this confirmed the Nernstian behaviour. Satisfactory correlation was obtained between the measured potentials and FUR concentration with the proposed sensors, and this was revealed by regression coefficient values of 0.9987 and 0.9980 for Sensors I and II, respectively. The LOD (limit of detection) values were calculated and reported for both the sensors. The experimental parameters were optimised to yield acceptable characteristics of both the sensors in the context of performance. The role of excipients of tablets and interferences were assessed by standard addition protocol. The obtained results confirmed the ineffective role of excipients of tablets and foreign species used as interferents.
Conclusion
The designed sensors were validated to confirm the accurate, precise, robust and rugged functioning in determining FUR. The mean of recovered FUR, close to 100%, revealed the acceptable and effective functioning of the proposed sensors. Excellent results were obtained by FUR tablets’ analysis using both the sensors.
Similar content being viewed by others
Background
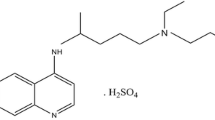
Frusemide or furosemide (FUR), chemically known as 4-chloro-2-[(furan-2-ylmethyl)-amino]-5-sulfamoyl-benzoic acid (Fig. 1), as a potent diuretic, is used to treat excessive fluid accumulation and swelling of the body caused by heart failure, cirrhosis, chronic kidney failure, and nephritic syndrome [1].
The drug FUR is monographed in United States Pharmacopeia [2] for assay. The procedure describes titration of FUR (about 600 mg) in dimethylformamide against standardised 0.1 M NaOH solution using bromothymol blue as indicator.
The literature survey revealed that several analytical techniques were employed to determine FUR in pharmaceuticals, and they include titrimetry [3, 4], ultraviolet (UV) spectrophotometry [5, 6], visible spectrophotometry [7,8,9,10,11,12,13,14,15,16,17,18,19,20], spectrofluorimetry [21,22,23], and high-pressure liquid chromatography (HPLC) technique with different detection systems like UV [24], fluorescence [25, 26], amperometric [27], diode array [28], and mass spectrometric (MS) [29]. Besides, gas chromatography–MS [30], micellar-LC [31], derivative spectroscopy with ratio-spectral analysis [32], and diffuse reflectance spectroscopic [33] techniques were also used for FUR.
A couple of reports were found in the literature on potentiometric sensors used to quantify FUR. Santini et al. [34] described the construction and analytical application of a potentiometric sensor using platinum, mercury, mercuric nitrate, and graphite to estimate FUR. The article devoted by Dias et al. [35] describes the construction and use of polymeric electrode by incorporating the FUR–tricaprylmethylammonium chloride (FUR-aliquat 336S) ion pair complex into a membrane for assaying FUR. The reported potentiometric sensors are not selective to determine FUR in pharmaceuticals because of their incomplete validation and scanty competing analytical results. Therefore, in this paper, the preparation, validation, and application of two new membrane sensors are presented for FUR determination in pharmaceuticals.
Methods
Apparatus
A PICO model digital potentiometer (Chennai-32, India) was used in all potential measurements. An Elico (Mumbai, India) pH metre was used in pH recordings. The electrochemical cell utilised Ag/AgCl as a reference electrode and a copper wire as a conducting device.
High-purity distilled water and analytical grade chemicals were used. The reagents were prepared using analytical grade substances. Jubilant Life Sciences Ltd, Nanjangud, Mysore, India gifted the pure FUR (99.8%), and the sample was used as received. Lasix tablets (40 mg FUR/tablet) were obtained from the local market. Bromophenol blue dye (BPB), bromocresol green (BCG), tetrahydrofuran (THF), chloroform (CHCl3), dibutyl phthalate (DBP), and polyvinyl chloride (PVC) were supplied from SD Fine Chem Ltd., Mumbai, India. Glacial acetic acid (HOAc) was purchased from Merck (Mumbai, India) and used to prepare a 2:3 (v/v) solution in water.
Solutions of 4 M sodium acetate (NaOAc) and 0.1% each of acetic acid (CH3COOH), sodium hydroxide (NaOH), sodium carbonate (Na2CO3), sodium bicarbonate (NaHCO3), ammonium acetate (CH3COONH4), potassium thiocyanate (KSCN), fructose, sucrose, maltose, glucose, lactose, starch, glycine, ammonium chloride (NH4Cl), cadmium chloride (CdCl2), calcium chloride (CaCl2), cobalt chloride (CoCl2), sodium fluoride (NaF), nickel chloride (NiCl2), potassium chloride (KCl), sodium formate, sodium benzoate, sodium salicylate, sodium citrate, sodium tartarate, sodium sulphate, sodium oxalate, and ammonium borate were prepared using pure compounds purchased from S.D. Fine Chem Pvt Ltd, in water.
A stock of 3 mmol/L FUR solution was prepared in 2:3 HOAc using a calibration flask.
General procedures
Designing of sensors
Accurately weighed 10.1 mg of FUR was dissolved in 25 mL 2:3 HOAc. To the same solution, 20.04 mg of BPB or 20.6 mg of BCG dye was added; the content was transferred into a separatory funnel and extracted thrice with 10 mL portions of chloroform. The organic layer was passed through anhydrous sodium sulphate and collected into a clean and dry beaker. After evaporating the solvent, the resulted residue in the form of ion pair was used. About 20.0 mg of FUR-BPB or FUR-BCG ion pair was dissolved in 10 mL THF. The solution took in a Petri dish (width 4 cm), 0.1 g each of DBP and PVC were added; it was dissolved, and the content was dried under laboratory temperature for 24 h. The obtained dry membrane was composed with 9% (w/w) of respective ion pair and 45.5% (w/w) each of DBP and PVC. The resultant sufficiently dried membrane was attached to one end of a plastic tube using THF and dried at room temperature for 24 h. A 3–5 mL internal solution of 0.005 mol/L FUR was filled, the lower terminal of copper wire (15-cm length and 2.0-mm diameter) coated with insulator (leaving 1.0 cm at the top end and 0.5 cm at the bottom not insulated) was immersed, and the upper terminal was taken into connection to a negative terminal of the potentiometer. At this moment, the resulted FUR-BPB (Sensor I) and FUR-BCG (Sensor II) sensors were soaked into FUR solution and were used for measuring potentials after 5 h.
Procedure for bulk drug
Construction of calibration curves
Using a microburet, different volumes (0.0, 0.08, 1.0, 2.0, 3.0, 4.0, 5.0, 6.0, 7.0, and 8.0 ml) of 3.0 mmol/L FUR solutions were availed in a series of 10-mL calibrated flasks. The volumes in each flask were raised to 8 mL with 2:3 HOAc, and the pH were adjusted to 3.71 with 4 M NaOAc solution. The volumes were brought to 10 mL with 2:3 HOAc and the potentials were measured with respective membrane sensor against Ag/AgCl reference electrode.
The calibration curves were constructed using the solutions’ potentials data versus log [FUR] were prepared. The FUR concentrations in unknowns were calculated using calibration curves or regression data derived using potential versus log [FUR].
Study of interference
A 2-ml each of 3 mmol/L FUR and 0.1% interferent solutions were taken in an array of 10-mL calibrated flasks. The solutions were made to the mark with 2:3 HOAc and the potentials of each measured using FUR-BPB (Sensor I) or FUR-BCG (Sensor II) indicative sensors in reference to Ag/AgCl standard electrode.
Analysis of tablets
A group of twenty pre-weighed tablets were ground into a fine powder. The homogeneous powder corresponding to 50 mg FUR was measured and taken in a 50-ml calibrated flask, added 20 ml HOAc and shaken for 20 min and the volume raised to calibration mark with water. The content after mixing was filtered with Whattman No. 41 paper. A suitably measured volume of the resultant solution was drawn out, and potential was measured as given under the calibration curve construction. The FUR concentration in the taken extract was computed.
Results
Optimisation of parameters
Effect of dye content
The quantity of BPB or BCG was varied in the preparation of ionic aggregate with FUR. Sensors prepared with differential amounts of dyes were employed to measure the potential, and consequently, slopes of the linear curves were found out. Calibration lines’ slopes were close to 59.16 mV/decade when the quantities of BPB and BCG added were 20.04 and 20.6 mg in Sensors I and II, respectively. At other quantities of dyes, varying slopes were observed. The resultant slopes from differential amounts of dyes are summarised in Table 1.
Response time and lifetime of the electrodes
The time required to achieve a steady-state potential (within ± 0.2 mV/min), for 2.4 × 10-5–2.4 × 10-3 mol/L FUR solutions at pH 3.71. The potentials were reached steady state in 8 s for FUR solutions at concentrations above 1.0 × 10-3 mol/L and about 20 s at concentrations near the limit of detection. The experimental results proved 60 days of lifetime with a total of 500 determinations using these FUR-BPB and FUR-BCG sensors. During this period, each sensor was in daily use and was stored in 0.010 mol/L FUR solution and pH adjusted at 3.71 when not in use. There were no significant changes in working concentration range, slope, and response time in this period.
Effect of plasticizer
The sensing membranes were prepared separately by adding different amounts of dibutyl sebacate (DBS), dibutyl phthalate (DBP), dioctyl phthalate (DOP), and o-nitrophenyl octylether (NPOE) as plasticizers. The membranes were prepared using 0.1 g of DBP for both; the sensors were found to behave in Nernstian response. The sensors were found to perform satisfactorily with respect to stable potential readings, ease of conditioning, and less response time. Therefore, DBP was used as plasticizer in fabricating the FUR-BPB and FUR-BCG membrane sensors.
pH effect
The pH effect on electrode potential using electrodes over the range of 2.0–8.0 for 2.4 × 10-5–2.4 × 10-3 mol/L FUR solutions was investigated. The resulting solutions’ pHs were adjusted with either 2 M HNO3 or dilute NaOAc solutions. As it can be seen in Fig. 2, the potential response was found maximum and acceptable at pH 3.71 for both the sensors. However, at pHs which differ from 3.71, significant change in either the solution’s clarity or potentials was observed. Therefore, the pH of 3.71 was maintained as optimum in all potential measurements.
Evaluation of selectivity coefficients
The chemical interaction between the active sites of the membrane surface and the analyte is responsible for generation of membrane potential (Em). The poor selectivity of membranes towards a single species is found due to the factor of chemical process on the signal. Practically, Emem is in proportion to the concentration ions capable of interacting at the membrane’s active sites. The generalised Nernst equation to include the contribution of an interferent, I, can be written as follows [36].
where K is a constant and includes reference electrode’s potential, liquid junction potential, and Em; ZA and ZI are the charges on the analyte and interferent, respectively, with concentrations [A] and [I]. The parameter KA,I is known as selectivity coefficient and is defined as
where [A]E and [I]E are the analyte’s and interferent’s concentrations yielding identical cell potentials, i.e. E. When the KA,I is equal to unity, the membrane responds for both analyte and interferent. A selective membrane must always possess KA,I value of less than unity [36].
Thus, as an important feature, the value of KA,I can be evaluated using the data of potentials of solutions contained with known but constant amount of interferent, [I]add, and varying amounts of analyte. Two distinct linear regions in the plot of Ecell against logarithmic values of the analyte’s concentration are very informative in evaluating the value of KA,I. When the [A] is significantly larger than KA,I [I]add, Ecell is a linear function of log [A]. If KA,I [I]add is significantly larger than [A]; however, the Ecell remains constant. The analyte’s and interferent’s concentrations at the intersection of the two linear regions are used to calculate KA,I.
Thus, KA,I values were calculated in the presence of different interferents and are given below in Table 2. Both the sensors were used to measure the potentials of FUR along with various inorganic and organic compounds, anions, and cations. A 0.1% solution of each interferent was spiked into pre-analyzed FUR solution, and analysis was done [37, 38]. The summarised results indicated non-interference from the added species as showed by the calculated KA,I values in Table 2. Therefore, the developed sensors are the most suitable electrodes for assay of FUR in the presence of such studied interferents, too.
Validation of sensors
The developed FUR-BPB and FUR-BCG sensors were evaluated for linearity by following the IUPAC [37, 38] recommendations. The sensors were validated for accuracy, precision, robustness, and ruggedness by following the ICH Guidelines [39]. The validation results for individual parameters are presented in the following sections.
Linearity and analytical and regression parameters
By following the IUPAC recommendations [37, 38], the linearity of the proposed analytical methods using FUR-BPB and FUR-BCG ISEs was evaluated. The results reflected that the ISEs respond rapidly and linearly for FUR concentration ranges presented in Table 3. The calibration graphs were linear (Fig. 3), and the regression equations were y = 58.73x + 322.8 and y = 57.65x + 336.89 for FUR-BPB and FUR-BCG ISEs, and the corresponding Nernstian slopes were 58.73 and 57.66 mV/decade. The regression coefficients, slopes, intercepts of the calibration lines and other relevant performance characteristics of both analytical methods using proposed sensors were calculated as described in the literature [37, 38, 40] and are mentioned in Table 3. The detection limits (LOD) were also calculated [37, 38] and reported in Table 3.
Intra- or same- and inter- or different-day precision and accuracy
Seven replicates each of three differently concentrated FUR solutions between the linear ranges were prepared; analyses were carried out on the same day and the %RSD values were calculated for intra- or same-day variations. However, inter- or between-day studies were done by analyzing five replicates of pure FUR solutions of three different concentrations during different days. The %RSD values were calculated for resultant FUR amounts. The accuracy was appraised by calculating the relative error (RE), the metric for accuracy, for each resultant concentration of FUR. The %RSD values of 1.13 to 3.11% declared the suitability of both sensors for precise determination of FUR. The accuracy was expressed using the metric of %RE and showed below in Table 4. The %RE values which ranged from 0.48 to 4.67 (Table 4) revealed the accurate performance of sensors. The sensors functioning without crossing the limits of accuracy range from 95.0 to 105.0% for pharmaceutical samples as recommended by ICH Guidelines [41] and USP [42] recommendations and made their applicability for use as quantification sensors for FUR.
Robustness and ruggedness
The robust functioning of proposed ISEs was assessed by varying working pH and temperatures slightly. FUR solutions of 0.9, 1.5, and 2.1 mmol/L were used for analysis. The pH was varied by 0.02 [3.71 (± 0.02)] units for each sensor, and the calculated values of %RSD were from 1.78 to 3.33. Besides, the robustness was also been evaluated by varying the temperature by 1 °C during the measurement of potentials of FUR solutions of different concentrations. The temperatures of the FUR solutions were brought to 23 ± 1 and 37 ± 1 °C using both the sensors; potentials were measured; and %RSD values were reported. Range of %RSD from 2.68 to 3.52 indicated the robust functioning of proposed sensors.
Analyses were done using three different potentiometers for 5 days by the same and different analysts to assess the ruggedness. The variations expressed in %RSD values for inter-potentiometric and inter-analysts were < 5%, reflecting sensors’ rugged function. Summary of results of this study are presented in Table 5.
Application of ISEs to tablets analysis
Five replicates of each of 0.9, 1.5 and 2.1 mmol/L in FUR from tablet extracts were analyzed with proposed Sensor I and Sensor II by using the described procedure for tablet analysis. The mean of the measured potentials obtained and the percent recoveries of FUR were evaluated. The results of the United States Pharmacopeia (USP) assay method [2] were compared with the obtained results using the proposed sensors. The procedure in reference method describes titration of FUR (about 600 mg) in dimethylformamide against standardised 0.1 M NaOH using bromothymol blue indicator. The Student’s t and and F values were intently obtained by calculation. The values of tcal and Fcal are tabulated in Table 6. The tcal and Fcal values are less than the values which are statistically tabulated. Therefore, the proposed sensors yielded accurate and precise results.
Recovery study
The accurate functioning of proposed ISEs was further assessed by recovery studies following the standard addition method. The aliquots of pre-analyzed tablet extracts were spiked with pure FUR solution in three different concentration levels. To a fixed amount of five replicates of FUR tablet extract, pure FUR in amounts corresponding to 50, 100, and 150% to that of the amount from tablets were spiked; pH was manipulated to optimum value, and content was diluted to 10 mL and potentials measured. Then, FUR concentrations were calculated using the regression data. The %FUR recovered was calculated for each level. The mean %FUR recovered was 98.33 with standard deviation of less than 3% revealed that good and acceptable recovery is possible with proposed sensors. The relevant results of recovery experiments are summarised below (Table 7).
Discussion
The development of ISEs using the ion pair complexes of therapeutic compounds and sulphonthalein dyes through their application to quantify FUR is described for the first time. The action of two sulphonthalein dyes viz. BPB and BCG is en-cashed to construct the membrane sensors or ISEs for FUR. Chemically, the nitrogenous group of FUR will possibly be utilised to react with anionic dye, as chromogenic reagent. In CHCl3, the resulting yellow product of FUR-BPB or FUR-BCG is due to the transformation of the lactoid ring structure to quinoid form [43] in the anionic dyes, BPB and BCG. The two tautomers (lactoid and quinoid) are supposed to be present in equilibrium. However, due to the acidic property of the sulphonic acid group, the latter (quinoid) form is assumed predominant. Finally, protonated FUR (FURH+) forms ion-pair with the anionic dye (BPB- or BCG-). These possible reaction mechanisms are presented in Scheme 1. Anionic dye such as BPB or BCG forms an ionic aggregate or ion pair complex with a positively charged drug, and the product formed between the drug and dye is an electrically neutral ion pair complex molecule. This formed the basis to develop novel membranes to fabricate potentiometric sensors of FUR with BPB and BCG in THF and PVC.
The acidic solution of FUR reacted with either BPB or BCG and formed a stable aqueous insoluble product of ion pair of yellow-coloured appropriate grain size. The probable structures of ion pair complexes are given below (Scheme 1). The aqueous insoluble ion pair complex of FUR-BPB or FUR-BCG is useful as a recombinant material to fabricate the membrane sensor. The membrane is formed effectively with uniform thickness all over its area when PVC is used as matrix and DBP is used as plasticizer. The membranes constructed here should therefore selectively respond to FUR, as the artificial ion-selective membrane sensors. The potentiometry will enable their use to confirm the selective functioning by generating the potential difference due to the varied concentrations of FUR solutions at opposite sides of the membrane [44].
The obtained product was used successfully to construct two sensors, namely FUR-BPB (Sensor I) and FUR-BCG (Sensor II) using THF as solvent and PVC. These acid–base indicator-based Sensor I and Sensor II were utilised for the first time to quantify FUR in pure form and in tablets.
The membranes developed were used to fabricate Sensors I and II and to measure the potentials of the drug solutions. The systematic representation of the electrochemical cell is represented as

Conclusion
For the first time, the two membrane sensors for potentiometric determination of frusemide using two acid dyes, namely bromophenol blue and bromocresol green are presented. The sensors are applicable to quantify frusemide over a wider linear range of concentration with the Nernestian response. The sensors have been successfully used to reliably determine frusemide in pure state and tablets. Good agreement evolved out of the results while using these sensors to determine frusemide accurately and precisely. Potentiometry with these sensors is the most selective technique to determine frusemide in pharmaceutical laboratories. Since, potentiometry is neither highly sophisticated nor have stringent steps to be followed; these could be used as a conventional technique in place of other high-cost techniques such as HPLC, LC-MS, GC-MS, etc.
Availability of data and materials
Data and material are available upon request.
Abbreviations
- BPB:
-
Bromophenol blue
- BCG:
-
Bromocresol
- FUR:
-
Frusemide
- THF:
-
Tetrahydrofuran
- PVC:
-
Polyvinyl chloride
- LOD:
-
Limit of detection
- Ag/AgCl:
-
Silver silver chloride
- FURH+ :
-
Protonated FUR
- BPB- :
-
Anionic BPB
- BCG- :
-
Anionic BCG
- Cu:
-
Copper
- SD:
-
Standard deviation
- RSD:
-
Relative standard deviation
- RE:
-
Relative error
References
Merck Index, 11th ed., Rahway, N.J. Merck, (1996) 4.
The United States Pharmacopoeial Convention, USP 35-NF 30, (2012) 3292-3293.
Basavaiah K, Chandrashekar U, Nagegowda P (2005) Rapid titrimetric and spectrophotometric determination of frusemide (furosemide) in formulations using bromate-bromide mixture and methyl orange. Indian J Chem Technol 12(2):149–155
Kulichenko SA, Fesenko SA (2002) Titrimetric determination of furosemide using aqueous–micellar solutions of surfactants. J Anal Chem 57(3):231–234
Dias ILT, Martins JLS, Neto GO (2005) Furosemide determination by first-derivative spectrophotometric method. Anal Lett 38(7):1159–1166
The United States Pharmacopoeial Convention. The United States pharmacopoeia XXIV. Rockville, MD, pp. 756 USA (2000).
Ljiljana Z, Snezana A, Dusanka R (1990) Spectrophotometric determination of furosemide as its Fe(III) complex in pharmaceutical preparations. Microchim Acta 100(1-2):49–54
Silva SF, Gomes CT (2006) Spectrophotometric determination of furosemide based on its complexation with Fe (III) in ethanolic medium using a flow injection procedure. Anal Lett 39:2557–2567
Mishra P, Katrolia D, Agrawal RK (1990) A simple colorimetric determination of furosemide in dosage forms. Indian J Pharm Sci 52(3):155–157
Golcu A (2006) Spectrophotometric determination of furosemide in pharmaceutical dosage forms using complex formation with Cu(II). J Anal Chem 61(8):748–754
Silva SF, Adelino DR, Gomes CT (2005) Flow injection spectrophotometric determination of furosemide in pharmaceuticals by the bleaching of a permanganate carrier solution. J Flow Inject Anal 22(1):34–37
Sevillano-Cabeza A, Campins-Falco P, Serrador-Garcia MC (1997) Extractive-spectrophotometric determination of furosemide with sodium 1,2-naphthoquinone-4-sulphonate in pharmaceutical formulations. Anal Lett 30(1):91–107
Garcia MS, Sanchez-Pedreno C, Albero MI, Rodenas V (1997) Flow-injection spectrophotometric determination of frusemide or sulphathiazole in pharmaceuticals. J Pharm Biomed Anal 15(4):453–459
Issopoulos PB (1989) Spectrophotometric determination of microquantities of frusemide using iso- and heteropolyanions of molybdenum(VI) as oxidizing agents. Fresenius J Anal Chem 334(6):554–557
Jasmin S, Rasuljan M, Khan MA (2005) Determination of furosemide by simple diazotization method in pharmaceutical preparations. J Chin Chem Soc 52(2):347–352
Sastry CSP, Tata NV, Sastry BS, Rao EV (1988) Spectrophotometric methods for the determination of some diuretics using 3-methyl-2-benzothiazolinone hydrazine. Analyst 113:255–258
Sastry CSP, Suryanarayana MV, Tipirneni ASRP (1989) Application of p-N,N-dimethylphenylenediamine dihydrochloride for the determination of some diuretics. Talanta 36(4):491–494
Tharpa K, Basavaiah K (2010) Use of a diazocoupling reaction for sensitive and selective spectrophotometric determination of furosemide in spiked human urine and pharmaceuticals. Chem Pap 64(4):415–423
Rani GD, Rani AR, Venkateswarlu P (2017) Spectrophotometric determination of Furosemide in pharmaceutical formulations by charge transfer complex method. Intl J ChemTech Res 10(3):666–670
Tharpa K, Basavaiah K, Vinay KB (2009) Spectrophotometric determination of furosemide in pharmaceuticals using permanganate. Jordan J Chem 4(4):387–397
Llorent-Martinez EJ, Ortega-Barrales P (2005) Multicommuted flow-through fluorescence optosensor for determination of furosemide and triamterene. Anal Bioanal Chem 383(5):797–803
Ioannou PC, Rusakoav NV, Andrikopoulou DA, Glynou KM, Tzompanaki GM (1998) Spectrofluorimetric determination of anthranilic acid derivatives based on terbium sensitized fluorescence. Analyst 123:2839–2843
Semaan FS, Nogueira PA, Cavalheiro ETG (2008) Flow-based fluorimetric determination of furosemide in pharmaceutical formulations and biological samples: use of micellar media to improve sensitivity. Anal Lett 41(1):66–79
Jankowshi A, Skorek-Jankowska A, Lamparczyk H (1997) Determination and pharmacokinetics of a furosemide-amiloride drug combination. J Chromatogr B Biomed Sci Appl 693(2):383–391
Carolina GG, Carlos PR, Gloria MGC, Rolando HR, Richardo RG (2005) Method validation for the determination of furosemide in plasma by liquid-liquid extraction and high-performance liquid chromatography with fluorescence detection. J Chil Chem Soc 50(2):479–482
Reeuwijk HJ, Tjaden UR, Vander JG (1992) Simultaneous determination of furosemide and amiloride in plasma using high-performance liquid chromatography with fluorescence detection. J Chromatogr B Biomed Sci Appl 575(2):269–274
Guzman A, Agui L, Pedrero M, Yanez-sedeno P, Pingarron JM (2003) Flow injection and HPLC determination of furosemide using pulsed amperometric detection at microelectrodes. J Pharm Biomed Anal 33(5):923–933
Semaan FS, Neto AJS, Lancas FM, Cavalheiro ETG (2005) Rapid HPLC-DAD determination of furosemide in tablets using a short home-made column. Anal Lett 38(10):1651–1658
Abdel-Hamid ME (2000) High-performance liquid chromatography–mass spectrometric analysis of furosemide in plasma and its use in pharmacokinetic studies. IL Farmaco 55(6-7):448–454
Ptacek P, Yhnalek OV, Breuel HP, Macek J (1996) Determination of furosemide in plasma and urine by gas chromatography/mass spectrometry. Arzneimittelforschung 46(3):277–283
Carda-Broch S, Esteve-Romero J, Ruiz-Angel MJ, Garcia-Alvarez-Coque MC (2002) Determination of furosemide in urine samples by direct injection in a micellar liquid chromatographic system. Analyst 127(1):29–34
Millership JS, Parker C, Donnelly D (2005) Ratio spectra derivative spectrophotometry for the determination of furosemide and spironolactone in a capsule formulation. IL Farmaco 60(4):333–338
Gotardo MA, Gigante AC, Pezza L, Pezza HR (2004) Determination of furosemide in pharmaceutical formulations by diffuse reflectance spectroscopy. Talanta 64(2):361–365
Santini AO, Pezza HR, Sequinel R, Rufino JL, Pezza L (2009) Potentiometric sensor for furosemide determination in pharmaceuticals, urine, blood serum and bovine milk. J Braz Chem Soc 20(1):64–53
Dias ILT, Neto GO, Vendramini DC, Sommer C, Martins JLS, Kubota LT (2004) A poly(vinyl chloride) membrane electrode for the determination of the diuretic furosemide. Anal Lett 37(1):35–46
Harvey D (2000) Textbook of modern analytical chemistry, 1st Edition, DePAUW University, International Edition, McGraw-Hill Companies, pp 477-478.
Buck R, Lindner E (2009) Recommendations for nomenclature of ion selective electrodes (IUPAC Recommendations 1994). Pure Appl Chem 66(12):2527–2536
International Union of Pure and Applied Chemistry: Analytical Chemistry Division, COMMISSION ON ELECTROANALYTICAL CHEMISTRY (2000) Potentiometric selectivity coefficients of ion selective electrodes. Part I. Inorganic cations. Pure Appl Chem 72(10):1851-2082.
International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use, ICH Harmonized Tripartite Guideline. Validation of analytical procedures: text and methodology Q2 (R1), complementary guideline on methodology. dated 06 November 1996, incorporated in November 2005, London.
Mamdouh RR, Adel MM, Hayam ML, Mostafa AS (2012) Validation of selective electrochemical method for determination of sumatriptan in combined dosage form. Anal Bioanal Electrochem 4(6):553–563
International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use. Validation of analytical procedures: text and methodology, Q2 (R1), 2005.
United States Pharmacopoeia 23 NF-18, Validation of compendial methods. The United States Pharmacopeial Convention, Rockville, 1995.
Ashour S, Fawaz CM, Bayram R (2006) Spectrophotometric determination of alfuzosin Hcl in pharmaceutical formulations with some sulphonephthalein dyes. Int J Biomed Sci 2(2):273–278
Harvey D (2000) Textbook of modern analytical chemistry, 1st Edition, DePAUW University, International Edition, McGraw-Hill Companies, pp 475.
Acknowledgements
Authors thank Jubilant Life Sciences Ltd, Nanjangud, Mysuru, India, for gifting pure furosemide drug for analyses. The first author is grateful to the JSS Mahavidyappetha, Mysuru and the Principal of JSS College of Arts, Commerce and Science, B N Road, Mysuru, India, for providing the facilities to pursue this work.
Funding
The work was funded for the project proposal to take up research on the development of new potentiometric sensors to determine some therapeutic compounds (Award No. 1495-MRP/14-15/KAMY013/UGC-SWRO, dated 04-02-15) by the University Grants Commission, New Delhi, India.
Author information
Authors and Affiliations
Contributions
NR and KB contributed equally to the work and in the overall work of manuscript preparation. Both authors read and approved the manuscript for further processing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rajendraprasad, N., Basavaiah, K. Application of ion pair complexes to design novel potentiometric membrane sensors for direct determination of frusemide in pharmaceuticals. Futur J Pharm Sci 6, 101 (2020). https://doi.org/10.1186/s43094-020-00081-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43094-020-00081-7