Abstract
Background
Fisetin, a plant active polyphenol, is well known for its antioxidant and free radical scavenging activities. The present study was designed to explore the detailed molecular mechanism underlying its neuroprotective effects.
Methods
The young male mice were either administered a single dose of lipopolysaccharide (0.83 mg/kg) or subjected to restraint stress (6 h per day for 28 days) to induce behavioral deficits in different groups. Fisetin (15 mg/kg) was orally administered for the last 14 days of the study.
Results
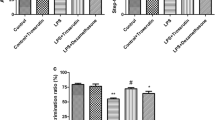
Lipopolysaccharide (LPS) as well as restraint stress (RS) exposure caused behavioral alterations (anxiety and depressive-like behavior). Gene expression analysis showed upregulation of nuclear Factor kappa-light-chain-enhancer of activated B cells (NF-κB) and indoleamine 2,3-dioxygenase (IDO)-1 gene expression along with downregulation of Nrf-2 (nuclear factor erythroid 2-related factor 2), HO-1 (heme oxygenase-1), and ChAT (choline acetyltransferase) gene expression level in RS and RS+LPS groups. Fisetin administration significantly ameliorated behavioral and neurochemical deficits in LPS, RS, and RS+LPS groups.
Conclusion
These findings clearly indicated that fisetin administration improved behavioral functions and suppressed the NF-κB and IDO-1 (indoleamine 2,3-dioxygenase) activation along with their antioxidant effect, suggesting fisetin as an intriguing nutraceutical for the management of inflammation-associated neurological disorders.






Similar content being viewed by others
References
Ahmed SMU, Luo L, Namani A et al (2017) Nrf2 signaling pathway: pivotal roles in inflammation. Biochim Biophys Acta - Mol Basis Dis 1863:585–597. https://doi.org/10.1016/j.bbadis.2016.11.005
André C, Dinel A-L, Ferreira G, Layé S, Castanon N (2014) Diet-induced obesity progressively alters cognition, anxiety-like behavior and lipopolysaccharide-induced depressive-like behavior: focus on brain indoleamine 2, 3-dioxygenase activation. Brain Behav Immun 41:10–21
Autry AE, Monteggia LM (2012) Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol Rev 64:238–258. https://doi.org/10.1124/pr.111.005108
Barbosa Filho JM, Medeiros KCP, de FFM DM et al (2006) Natural products inhibitors of the enzyme acetylcholinesterase. Rev Bras Farmacogn 16:258–285
Bathina S, Das UN (2015) Brain-derived neurotrophic factor and its clinical implications. Arch Med Sci 11:1164–1178. https://doi.org/10.5114/aoms.2015.56342
Beutler E (1963) Improved method for the determination of blood glutathione. J Lab Clin Med 61:882–888
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Breese GR, Knapp DJ, Overstreet DH, Navarro M, Wills TA, Angel RA (2008) Repeated lipopolysaccharide (LPS) or cytokine treatments sensitize ethanol withdrawal-induced anxiety-like behavior. Neuropsychopharmacology 33:867–876
Capiotti KM, De Moraes DA, Menezes FP et al (2014) Hyperglycemia induces memory impairment linked to increased acetylcholinesterase activity in zebrafish (Danio rerio). Behav Brain Res 274:319–325. https://doi.org/10.1016/j.bbr.2014.08.033
Chang H-A, Wang Y-H, Tung C-S, Yeh CB, Liu YP (2016) 7,8-Dihydroxyflavone, a tropomyosin-kinase related receptor B agonist, produces fast-onset antidepressant-like effects in rats exposed to chronic mild stress. Psychiatry Investig 13:531–540. https://doi.org/10.4306/pi.2016.13.5.531
Conrad CD (2008) Chronic stress-induced hippocampal vulnerability: the glucocorticoid vulnerability hypothesis. Rev Neurosci 19:395–412
Currais A, Prior M, Dargusch R, Armando A, Ehren J, Schubert D, Quehenberger O, Maher P (2014) Modulation of p25 and inflammatory pathways by fisetin maintains cognitive function in Alzheimer’s disease transgenic mice. Aging Cell 13:379–390
Dwivedi DK, Jena GB (2018) Glibenclamide protects against thioacetamide-induced hepatic damage in Wistar rat: investigation on NLRP3, MMP-2, and stellate cell activation. Naunyn Schmiedebergs Arch Pharmacol 1–18
Feng G, Jiang Z, Sun B, Fu J, Li TZ (2016) Fisetin alleviates lipopolysaccharide-induced acute lung injury via TLR4-mediated NF-κB signaling pathway in rats. Inflammation 39:148–157
Fujigaki H, Saito K, Fujigaki S, Takemura M, Sudo K, Ishiguro H, Seishima M (2006) The signal transducer and activator of transcription 1α and interferon regulatory factor 1 are not essential for the induction of indoleamine 2, 3-dioxygenase by lipopolysaccharide: involvement of p38 mitogen-activated protein kinase and nuclear factor-κB. J Biochem 139:655–662
German-Ponciano LJ, Rosas-Sánchez GU, Rivadeneyra-Domínguez E, Rodríguez-Landa JF (2018) Advances in the preclinical study of some flavonoids as potential antidepressant agents. Scientifica (Cairo) 2018
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem 126:131–138
Guan Z, Fang J (2006) Peripheral immune activation by lipopolysaccharide decreases neurotrophins in the cortex and hippocampus in rats. Brain Behav Immun 20:64–71
Guo Y, Sun J, Li T, Zhang Q, Bu S, Wang Q, Lai D (2017) Melatonin ameliorates restraint stress-induced oxidative stress and apoptosis in testicular cells via NF-kappaB/iNOS and Nrf2/ HO-1 signaling pathway. Sci Rep 7:9599. https://doi.org/10.1038/s41598-017-09943-2
Gupta SC, Prasad S, Aggarwal BB (2016) Anti-inflammatory nutraceuticals and chronic diseases. Springer
Gutierrez-Merino C, Lopez-Sanchez C, Lagoa R, K. Samhan-Arias A, Bueno C, Garcia-Martinez V (2011) Neuroprotective actions of flavonoids. Curr Med Chem 18:1195–1212
Heisler JM, O’Connor JC (2015) Indoleamine 2, 3-dioxygenase-dependent neurotoxic kynurenine metabolism mediates inflammation-induced deficit in recognition memory. Brain Behav Immun 50:115–124
Huang G-B, Zhao T, Muna SS, Bagalkot TR, Jin HM, Chae HJ, Chung YC (2013) Effects of chronic social defeat stress on behaviour, endoplasmic reticulum proteins and choline acetyltransferase in adolescent mice. Int J Neuropsychopharmacol 16:1635–1647
Huang Y, Henry CJ, Dantzer R, Johnson RW, Godbout JP (2008) Exaggerated sickness behavior and brain proinflammatory cytokine expression in aged mice in response to intracerebroventricular lipopolysaccharide. Neurobiol Aging 29:1744–1753
Ikonomovic MD, Mufson EJ, Wuu J, Cochran EJ, Bennett DA, DeKosky ST (2003) Cholinergic plasticity in hippocampus of individuals with mild cognitive impairment: correlation with Alzheimer’s neuropathology. J Alzheimers Dis 5:39–48
Jangra A, Dwivedi S, Sriram CS, Gurjar SS, Kwatra M, Sulakhiya K, Baruah CC, Lahkar M (2016a) Honokiol abrogates chronic restraint stress-induced cognitive impairment and depressive-like behaviour by blocking endoplasmic reticulum stress in the hippocampus of mice. Eur J Pharmacol 770:25–32
Jangra A, Kwatra M, Singh T, Pant R, Kushwah P, Sharma Y, Saroha B, Datusalia AK, Bezbaruah BK (2016b) Piperine augments the protective effect of curcumin against lipopolysaccharide-induced neurobehavioral and neurochemical deficits in mice. Inflammation 39:1025–1038
Jangra A, Lukhi MM, Sulakhiya K, Baruah CC, Lahkar M (2014) Protective effect of mangiferin against lipopolysaccharide-induced depressive and anxiety-like behaviour in mice. Eur J Pharmacol 740:337–345
Jangra A, Sriram CS, Dwivedi S, Gurjar SS, Hussain MI, Borah P, Lahkar M (2017) Sodium phenylbutyrate and edaravone abrogate chronic restraint stress-induced behavioral deficits: implication of oxido-nitrosative, endoplasmic reticulum stress cascade, and neuroinflammation. Cell Mol Neurobiol 37:65–81
Jangra A, Sriram CS, Lahkar M (2016c) Lipopolysaccharide-induced behavioral alterations are alleviated by sodium phenylbutyrate via attenuation of oxidative stress and neuroinflammatory cascade. Inflammation 39:1441–1452
Kasbe P, Jangra A, Lahkar M (2015) Mangiferin ameliorates aluminium chloride-induced cognitive dysfunction via alleviation of hippocampal oxido-nitrosative stress, proinflammatory cytokines and acetylcholinesterase level. J Trace Elem Med Biol 31:107–112
Lacosta S, Merali Z, Anisman H (1999) Behavioral and neurochemical consequences of lipopolysaccharide in mice: anxiogenic-like effects. Brain Res 818:291–303
Lu Y-C, Yeh W-C, Ohashi PS (2008) LPS/TLR4 signal transduction pathway. Cytokine:42–145, 151. https://doi.org/10.1016/j.cyto.2008.01.006
Maher P (2009) Modulation of multiple pathways involved in the maintenance of neuronal function during aging by fisetin. Genes Nutr 4:297–307
Maher P (2015) Fisetin acts on multiple pathways to reduce the impact of age and disease on CNS function. Front Biosci (Schol Ed) 7:58–82
Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B (2007) Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet 370:851–858
Nunes-Tavares N, Santos LE, Stutz B, Brito-Moreira J, Klein WL, Ferreira ST, de Mello FG (2012) Inhibition of choline acetyltransferase as a mechanism for cholinergic dysfunction induced by amyloid-beta peptide oligomers. J Biol Chem 287:19377–19385. https://doi.org/10.1074/jbc.M111.321448
O’Connor JC, Lawson MA, Andre C et al (2009a) Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry 14:511–522. https://doi.org/10.1038/sj.mp.4002148
O’Connor JC, Lawson MA, André C et al (2009b) Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry 14:511–522. https://doi.org/10.1038/sj.mp.4002148
O’Connor JC, Lawson MA, André C et al (2009c) Induction of IDO by bacille Calmette-Guerin is responsible for development of murine depressive-like behavior. J Immunol 182:3202–3212
O’Keane V (2000) Evolving model of depression as an expression of multiple interacting risk factors. Br J Psychiatry 177:482–483. https://doi.org/10.1192/bjp.177.6.482
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Ohta Y, Kubo H, Yashiro K, Ohashi K, Tsuzuki Y, Wada N, Yamamoto Y, Saito K (2017) Effect of water-immersion restraint stress on tryptophan catabolism through the kynurenine pathway in rat tissues. J Physiol Sci 67:361–372. https://doi.org/10.1007/s12576-016-0467-y
Pant R, Jangra A, Kwatra M, Singh T, Kushwah P, Bezbaruah BK, Gurjar SS, Phukan S (2017) Cognitive deficits induced by combined exposure of stress and alcohol mediated through oxidative stress-PARP pathway in the hippocampus. Neurosci Lett 653:208–214
Park H-J, Shim HS, Kim H, Kim KS, Lee H, Hahm DH, Shim I (2010) Effects of glycyrrhizae radix on repeated restraint stress-induced neurochemical and behavioral responses. Korean J Physiol Pharmacol Off J Korean Physiol Soc Korean Soc Pharmacol 14:371–376. https://doi.org/10.4196/kjpp.2010.14.6.371
Prakash D, Gopinath K, Sudhandiran G (2013) Fisetin enhances behavioral performances and attenuates reactive gliosis and inflammation during aluminum chloride-induced neurotoxicity. NeuroMolecular Med 15:192–208
Prakash D, Sudhandiran G (2015) Dietary flavonoid fisetin regulates aluminium chloride-induced neuronal apoptosis in cortex and hippocampus of mice brain. J Nutr Biochem 26:1527–1539
Rajput P, Jangra A, Kwatra M, Mishra A, Lahkar M (2017) Alcohol aggravates stress-induced cognitive deficits and hippocampal neurotoxicity: protective effect of melatonin. Biomed Pharmacother 91:457–466
Rinne JO, Kaasinen V, Järvenpää T et al (2003) Brain acetylcholinesterase activity in mild cognitive impairment and early Alzheimer’s disease. J Neurol Neurosurg & Psychiatry 74:113 LP–113115
Sahu BD, Kalvala AK, Koneru M, Mahesh Kumar J, Kuncha M, Rachamalla SS, Sistla R (2014) Ameliorative effect of fisetin on cisplatin-induced nephrotoxicity in rats via modulation of NF-κB activation and antioxidant defence. PLoS One 9:e105070
Sahu BD, Kumar JM, Sistla R (2016) Fisetin, a dietary flavonoid, ameliorates experimental colitis in mice: relevance of NF-κB signaling. J Nutr Biochem 28:171–182
Segerstrom SC, Miller GE (2004) Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol Bull 130:601–630
Shih R-H, Wang C-Y, Yang C-M (2015) NF-kappaB signaling pathways in neurological inflammation: a mini review. Front Mol Neurosci 8:77
Sjöholm L, Lavebratt C, Forsell Y (2009) A multifactorial developmental model for the etiology of major depression in a population-based sample. J Affect Disord 113:66–76
Solanki I, Parihar P, Mansuri ML, Parihar MS (2015) Flavonoid-based therapies in the early management of neurodegenerative diseases. Adv Nutr 6:64–72. https://doi.org/10.3945/an.114.007500
Sonawalla SB, Farabaugh A, Johnson MW, Morray M, Delgado ML, Pingol MG, Rosenbaum JF, Fava M (2002) Fluoxetine treatment of depressed patients with comorbid anxiety disorders. J Psychopharmacol 16:215–219
Sriram CS, Jangra A, Gurjar SS, Hussain MI, Borah P, Lahkar M, Mohan P, Bezbaruah BK (2015) Poly (ADP-ribose) polymerase-1 inhibitor, 3-aminobenzamide pretreatment ameliorates lipopolysaccharide-induced neurobehavioral and neurochemical anomalies in mice. Pharmacol Biochem Behav 133:83–91
Steru L, Chermat R, Thierry B, Simon P (1985) The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology 85:367–370
Sulakhiya K, Kumar P, Jangra A, Dwivedi S, Hazarika NK, Baruah CC, Lahkar M (2014) Honokiol abrogates lipopolysaccharide-induced depressive like behavior by impeding neuroinflammation and oxido-nitrosative stress in mice. Eur J Pharmacol 744:124–131
Ueyama T, Kawai Y, Nemoto K, Sekimoto M, Toné S, Senba E (1997) Immobilization stress reduced the expression of neurotrophins and their receptors in the rat brain. Neurosci Res 28:103–110
Vijayaraghavan S, Karami A, Aeinehband S, Behbahani H, Grandien A, Nilsson B, Ekdahl KN, Lindblom RPF, Piehl F, Darreh-Shori T (2013) Regulated extracellular choline acetyltransferase activity—the plausible missing link of the distant action of acetylcholine in the cholinergic anti-inflammatory pathway. PLoS One 8:e65936. https://doi.org/10.1371/journal.pone.0065936
Wahba ZZ, Soliman KFA (1992) Effect of stress on choline acetyltransferase activity of the brain and the adrenal of the rat. Experientia 48:265–268
Wang Y, Lawson MA, Dantzer R, Kelley KW (2010) LPS-induced indoleamine 2, 3-dioxygenase is regulated in an interferon-γ-independent manner by a JNK signaling pathway in primary murine microglia. Brain Behav Immun 24:201–209
Wang Y, Xu J, Liu Y, et al (2018) TLR4-NF-κB signal involved in depressive-like behaviors and cytokine expression of frontal cortex and hippocampus in stressed C57BL/6 and ob/ob mice. Neural Plast 2018:
Wardyn JD, Ponsford AH, Sanderson CM (2015) Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem Soc Trans 43:621–626. https://doi.org/10.1042/BST20150014
WHO (2018) Depression key facts
Wichers MC, Maes M (2004) The role of indoleamine 2, 3-dioxygenase (IDO) in the pathophysiology of interferon-α-induced depression. J Psychiatry Neurosci 29:11
Wu P-Y, Lyu J-L, Liu Y-J, Chien TY, Hsu HC, Wen KC, Chiang HM (2017) Fisetin regulates Nrf2 expression and the inflammation-related signaling pathway to prevent UVB-induced skin damage in hairless mice. Int J Mol Sci 18:2118
Xu W, Zheng D, Liu Y, Li J, Yang L, Shang X (2017) Glaucocalyxin B alleviates lipopolysaccharide-induced Parkinson’s disease by inhibiting TLR/NF-kappaB and activating Nrf2/HO-1 pathway. Cell Physiol Biochem 44:2091–2104. https://doi.org/10.1159/000485947
Yu X, Jiang X, Zhang X, Chen Z, Xu L, Chen L, Wang G, Pan J (2016) The effects of fisetin on lipopolysaccharide-induced depressive-like behavior in mice. Metab Brain Dis 31:1011–1021
Zhang J, Yao W, Dong C, Yang C, Ren Q, Ma M, Han M, Hashimoto K (2015) Comparison of ketamine, 7,8-dihydroxyflavone, and ANA-12 antidepressant effects in the social defeat stress model of depression. Psychopharmacology 232:4325–4335. https://doi.org/10.1007/s00213-015-4062-3
Zhang M-W, Zhang S-F, Li Z-H, Han F (2016) 7,8-Dihydroxyflavone reverses the depressive symptoms in mouse chronic mild stress. Neurosci Lett 635:33–38. https://doi.org/10.1016/j.neulet.2016.10.035
Zhao X, Wang C, Cui W-G, Ma Q, Zhou WH (2015a) Fisetin exerts antihyperalgesic effect in a mouse model of neuropathic pain: engagement of spinal serotonergic system. Sci Rep 5:9043. https://doi.org/10.1038/srep09043
Zhao Y, Zhang Y, Pan F (2015b) The effects of EGb761 on lipopolysaccharide-induced depressive-like behaviour in C57BL/6J mice. Cent J Immunol 40:11–17. https://doi.org/10.5114/ceji.2015.49427
Zhen L, Zhu J, Zhao X, Huang W, An Y, Li S, du X, Lin M, Wang Q, Xu Y, Pan J (2012) The antidepressant-like effect of fisetin involves the serotonergic and noradrenergic system. Behav Brain Res 228:359–366. https://doi.org/10.1016/j.bbr.2011.12.017
Acknowledgements
The authors are also grateful to SBT Hub, College of Veterinary Sciences, Guwahati for providing technical support.
Funding
Authors would like to thank the National Institute of Pharmaceutical Education and Research (NIPER), Guwahati for financial assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All the experiments were carried out in accordance with the regulation of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India guidelines.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Choubey, P., Kwatra, M., Pandey, S.N. et al. Ameliorative effect of fisetin against lipopolysaccharide and restraint stress-induced behavioral deficits via modulation of NF-κB and IDO-1. Psychopharmacology 236, 741–752 (2019). https://doi.org/10.1007/s00213-018-5105-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-018-5105-3