Abstract
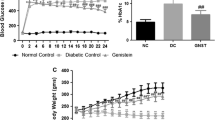
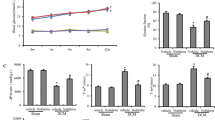
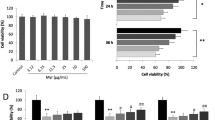
Long-standing diabetes is associated with increased oxidative stress and cardiac fibrosis. This, in turn, contributes to the progression of cardiomyopathy. The present study was sought to investigate whether the free radical scavenger, 4-hydroxy-2,2,6,6-tetramethyl piperidinoxyl (tempol) can protect against diabetic cardiomyopathy and to explore the specific underlying mechanism(s) in this setting. Diabetes was induced in rats by a single intraperitoneal injection dose of streptozotocin (50 mg/kg). These animals were treated with tempol (18 mg kg−1 day−1, orally) for 8 weeks. Our results showed significant increases in collagen IV and fibronectin protein levels and a marked decrease in matrix metalloproteinase-2 (MMP-2) activity measured by gelatin-gel zymography alongside elevated cardiac transforming growth factor (TGF)-β level determined using ELISA or immunohistochemistry in cardiac tissues of diabetic rats compared with control. This was accompanied by an increased in the oxidative stress as evidenced by increased reactive oxygen species (ROS) production and decreased antioxidant enzyme capacity along with elevated lactate dehydrogenase (LDH) and creatine kinase (CK-MB) serum levels as compared with the control. Tempol treatment significantly corrected the changes in the cardiac extracellular matrix, TGF-β, ROS or serum LDH, CK-MB levels, and normalized MMP-2 activity along with preservation of cardiac tissues integrity of diabetic rats against damaging responses. Moreover, tempol normalized the elevated systolic blood pressure and improved some cardiac functions in diabetic rats. Collectively, our data suggest a potential protective role of tempol against diabetes-associated cardiac fibrosis in rats via reducing oxidative stress and extracellular matrix remodeling.






Similar content being viewed by others
References
Ahmed SH, Clark LL, Pennington WR, Webb CS, Bonnema DD, Leonardi AH, McClure CD, Spinale FG, Zile MR (2006) Matrix metalloproteinases/tissue inhibitors of metalloproteinases: relationship between changes in proteolytic determinants of matrix composition and structural, functional, and clinical manifestations of hypertensive heart disease. Circulation 113:2089–2096
Bergman MR, Teerlink JR, Mahimkar R, Li L, Zhu BQ, Nguyen A, Dahi S, Karliner JS, Lovett DH (2007) Cardiac matrix metalloproteinase-2 expression independently induces marked ventricular remodeling and systolic dysfunction. Am J Physiol Heart Circ Physiol 292:H1847–H1860
Bollano E, Omerovic E, Svensson H, Waagstein F, Fu M (2007) Cardiac remodeling rather than disturbed myocardial energy metabolism is associated with cardiac dysfunction in diabetic rats. Int J Cardiol 114:195–201
Castro MM, Rizzi E, Rodrigues GJ, Ceron CS, Bendhack LM, Gerlach RF, Tanus-Santos JE (2009) Antioxidant treatment reduces matrix metalloproteinase-2-induced vascular changes in renovascular hypertension. Free Radic Biol Med 46:1298–1307
Coskun O, Kanter M, Korkmaz A, Oter S (2005) Quercetin, a flavonoid antioxidant, prevents and protects streptozotocin-induced oxidative stress and beta-cell damage in rat pancreas. Pharmacol Res 51:117–123
Date MO, Morita T et al (2002) The antioxidant N-2-mercaptopropionyl glycine attenuates left ventricular hypertrophy in in vivo murine pressure-overload model. J Am Coll Cardiol 39:907–912
Dhalla AK, Hill MF, Singal PK (1996) Role of oxidative stress in transition of hypertrophy to heart failure. J Am Coll Cardiol 28:506–514
Dobrian AD, Schriver SD, Prewitt RL (2001) Role of angiotensin II and free radicals in blood pressure regulation in a rat model of renal hypertension. Hypertension 38:361–366
El-Bassossy HM, Fahmy A, Badawy D (2011) Cinnamaldehyde protects from the hypertension associated with diabetes. Food Chem Toxicol 49:3007–3012
Fang ZY, Prins JB, Marwick TH (2004) Diabetic cardiomyopathy: evidence, mechanisms, and therapeutic implications. Endocr Rev 25:543–567
Feldman AM, Combes A, Wagner D, Kadakomi T, Kubota T, Li YY, McTiernan C (2000) The role of tumor necrosis factor in the pathophysiology of heart failure. J Am Coll Cardiol 35:537–544
Frustaci A, Kajstura J, Chimenti C, Jakoniuk I, Leri A, Maseri A, Nadal-Ginard B, Anversa P (2000) Myocardial cell death in human diabetes. Circ Res 87:1123–1132
Gohlke P, Kuwer I, Bartenbach S, Schnell A, Unger T (1994) Effect of low-dose treatment with perindopril on cardiac function in stroke-prone spontaneously hypertensive rats: role of bradykinin. J Cardiovasc Pharmacol 24:462–469
Goyal RK, Satia MC, Bangaru RA, Gandhi TP (1998) Effect of long-term treatment with enalapril in streptozotocin diabetic and DOCA hypertensive rats. J Cardiovasc Pharmacol 32:317–322
Hayashidani S, Tsutsui H, Ikeuchi M, Shiomi T, Matsusaka H, Kubota T, Imanaka-Yoshida K, Itoh T, Takeshita A (2003) Targeted deletion of MMP-2 attenuates early LV rupture and late remodeling after experimental myocardial infarction. Am J Physiol Heart Circ Physiol 285:H1229–H1235
Jarrett RJ (1989) Cardiovascular disease and hypertension in diabetes mellitus. Diabetes Metab Rev 5:547–558
Jesmin S, Sakuma I, Hattori Y, Fujii S, Kitabatake A (2002) Long-acting calcium channel blocker benidipine suppresses expression of angiogenic growth factors and prevents cardiac remodelling in a type II diabetic rat model. Diabetologia 45:402–415
Kodavanti UP, Schladweiler MC, Gilmour PS, Wallenborn JG, Mandavilli BS, Ledbetter AD, Christiani DC, Runge MS, Karoly ED, Costa DL, Peddada S, Jaskot R, Richards JH, Thomas R, Madamanchi NR, Nyska A (2008) The role of particulate matter-associated zinc in cardiac injury in rats. Environ Health Perspect 116:13–20
Kusaka M, Kishi K, Sokabe H (1987) Does so-called streptozocin hypertension exist in rats? Hypertension 10:517–521
Li C, Jackson RM (2002) Reactive species mechanisms of cellular hypoxia-reoxygenation injury. Am J Physiol Cell Physiol 282:C227–C241
Luo ZF, Feng B et al (2010) Effects of 4-phenylbutyric acid on the process and development of diabetic nephropathy induced in rats by streptozotocin: regulation of endoplasmic reticulum stress-oxidative activation. Toxicol Appl Pharmacol 246:49–57
Ma G, Al-Shabrawey M, Johnson JA, Datar R, Tawfik HE, Guo D, Caldwell RB, Caldwell RW (2006) Protection against myocardial ischemia/reperfusion injury by short-term diabetes: enhancement of VEGF formation, capillary density, and activation of cell survival signaling. Naunyn Schmiedebergs Arch Pharmacol 373:415–427
Ma F, Li Y, Jia L, Han Y, Cheng J, Li H, Qi Y, Du J (2012) Macrophage-stimulated cardiac fibroblast production of IL-6 is essential for TGF beta/Smad activation and cardiac fibrosis induced by angiotensin II. PLoS One 7:e35144
Martin J, Kelly DJ, Mifsud SA, Zhang Y, Cox AJ, See F, Krum H, Wilkinson-Berka J, Gilbert RE (2005) Tranilast attenuates cardiac matrix deposition in experimental diabetes: role of transforming growth factor-beta. Cardiovasc Res 65:694–701
Massague J, Chen YG (2000) Controlling TGF-beta signaling. Genes Dev 14:627–644
Mihm MJ, Seifert JL, Coyle CM, Bauer JA (2001) Diabetes related cardiomyopathy time dependent echocardiographic evaluation in an experimental rat model. Life Sci 69:527–542
Norby FL, Wold LE, Duan J, Hintz KK, Ren J (2002) IGF-I attenuates diabetes-induced cardiac contractile dysfunction in ventricular myocytes. Am J Physiol Endocrinol Metab 283:E658–E666
Pauschinger M, Knopf D, Petschauer S, Doerner A, Poller W, Schwimmbeck PL, Kuhl U, Schultheiss HP (1999) Dilated cardiomyopathy is associated with significant changes in collagen type I/III ratio. Circulation 99:2750–2756
Ren J, Davidoff AJ (1997) Diabetes rapidly induces contractile dysfunctions in isolated ventricular myocytes. Am J Physiol 272:H148–H158
Rodrigues B, Goyal RK, McNeill JH (1986) Effects of hydralazine on streptozotocin-induced diabetic rats: prevention of hyperlipidemia and improvement in cardiac function. J Pharmacol Exp Ther 237:292–299
Sano M, Fukuda K et al (2001) ERK and p38 MAPK, but not NF-kappaB, are critically involved in reactive oxygen species-mediated induction of IL-6 by angiotensin II in cardiac fibroblasts. Circ Res 89:661–669
Schnackenberg CG, Welch WJ, Wilcox CS (1998) Normalization of blood pressure and renal vascular resistance in SHR with a membrane-permeable superoxide dismutase mimetic: role of nitric oxide. Hypertension 32:59–64
Shimizu M, Umeda K, Sugihara N, Yoshio H, Ino H, Takeda R, Okada Y, Nakanishi I (1993) Collagen remodelling in myocardia of patients with diabetes. J Clin Pathol 46:32–36
Sowers JR, Epstein M (1995) Diabetes mellitus and associated hypertension, vascular disease, and nephropathy. An update. Hypertension 26:869–879
Sun Y, Zhang J, Lu L, Bedigian MP, Robinson AD, Weber KT (2004) Tissue angiotensin II in the regulation of inflammatory and fibrogenic components of repair in the rat heart. J Lab Clin Med 143:41–51
Taye A, Saad AH, Kumar AH, Morawietz H (2010) Effect of apocynin on NADPH oxidase-mediated oxidative stress-LOX-1-eNOS pathway in human endothelial cells exposed to high glucose. Eur J Pharmacol 627:42–48
Tian L, Li C, Qi J, Fu P, Yu X, Li X, Cai L (2008) Diabetes-induced upregulation of urotensin II and its receptor plays an important role in TGF-beta1-mediated renal fibrosis and dysfunction. Am J Physiol Endocrinol Metab 295:E1234–E1242
Torre-Amione G, Kapadia S, Benedict C, Oral H, Young JB, Mann DL (1996) Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: a report from the Studies of Left Ventricular Dysfunction (SOLVD). J Am Coll Cardiol 27:1201–1206
Tschope C, Walther T et al (2005) Transgenic activation of the kallikrein-kinin system inhibits intramyocardial inflammation, endothelial dysfunction and oxidative stress in experimental diabetic cardiomyopathy. FASEB J 19:2057–2059
Van Linthout S, Seeland U et al (2008) Reduced MMP-2 activity contributes to cardiac fibrosis in experimental diabetic cardiomyopathy. Basic Res Cardiol 103:319–327
Visse R, Nagase H (2003) Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res 92:827–839
Wang P, Li HW, Wang YP, Chen H, Zhang P (2009) Effects of recombinant human relaxin upon proliferation of cardiac fibroblast and synthesis of collagen under high glucose condition. J Endocrinol Invest 32:242–247
Westermann D, Rutschow S et al (2007) Contributions of inflammation and cardiac matrix metalloproteinase activity to cardiac failure in diabetic cardiomyopathy: the role of angiotensin type 1 receptor antagonism. Diabetes 56:641–646
Yamamoto J (1988) Blood pressure and metabolic effects of streptozotocin in Wistar–Kyoto and spontaneously hypertensive rats. Clin Exp Hypertens A 10:1065–1083
Yu W, Wu J, Cai F, Xiang J, Zha W, Fan D, Guo S, Ming Z, Liu C (2012) Curcumin alleviates diabetic cardiomyopathy in experimental diabetic rats. PLoS One 7:e52013
Zhao W, Zhao T, Chen Y, Ahokas RA, Sun Y (2008) Oxidative stress mediates cardiac fibrosis by enhancing transforming growth factor-beta1 in hypertensive rats. Mol Cell Biochem 317:43–50
Zile MR, Brutsaert DL (2002) New concepts in diastolic dysfunction and diastolic heart failure: Part I: diagnosis, prognosis, and measurements of diastolic function. Circulation 105:1387–1393
Acknowledgments
The authors are grateful to Prof. Adel M. Bakeer, Professor of Pathology, Faculty of Veterinary Medicine, Cairo University for his kind help in performing histopathological studies and interpretation of the results.
Conflict of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Taye, A., Abouzied, M.M. & Mohafez, O.M.M. Tempol ameliorates cardiac fibrosis in streptozotocin-induced diabetic rats: role of oxidative stress in diabetic cardiomyopathy. Naunyn-Schmiedeberg's Arch Pharmacol 386, 1071–1080 (2013). https://doi.org/10.1007/s00210-013-0904-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-013-0904-x