Abstract
Purpose
Mortality in circulatory shock is high. Enhanced resolution of shock may improve outcomes. We aim to determine whether adding hemodynamic monitoring with continual transesophageal echocardiography (hTEE) to usual care accelerates resolution of hemodynamic instability.
Methods
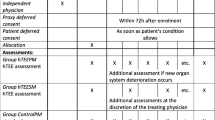
550 patients with circulatory shock were randomly assigned to four groups stratified using hTEE (hTEE vs usual care) and assessment frequency (minimum every 4 h vs 8 h). Primary outcome was time to resolution of hemodynamic instability, analyzed as intention-to-treat (ITT) analysis at day 6 and in a predefined secondary analysis at days 3 and 28.
Results
Of 550 randomized patients, 271 with hTEE and 274 patients with usual care were eligible and included in the ITT analysis. Time to resolution of hemodynamic instability did not differ within the first 6 days [hTEE vs usual care adjusted sub-hazard ratio (SHR) 1.20, 95% confidence interval (CI) 0.98–1.46, p = 0.067]. Time to resolution of hemodynamic instability during the 72 h of hTEE monitoring was shorter in patients with TEE (hTEE vs usual care SHR 1.26, 95% CI 1.02–1.55, p = 0.034). Assessment frequency had no influence. Time to resolution of clinical signs of hypoperfusion, duration of organ support, length of stay and mortality in the intensive care unit and hospital, and mortality at 28 days did not differ between groups.
Conclusions
In critically ill patients with shock, hTEE monitoring or hemodynamic assessment frequency did not influence resolution of hemodynamic instability or mortality within the first 6 days.
Trial registration and statistical analysis plan
ClinicalTrials.gov Identifier: NCT02048566.


Similar content being viewed by others
Change history
22 July 2019
The original version of this article unfortunately contained a mistake.
References
Vincent JL, De Backer D (2013) Circulatory shock. N Engl J Med 369:1726–1734
Martin C, Medam S, Antonini F, Alingrin J, Haddam M, Hammad E, Meyssignac B, Vigne C, Zieleskiewicz L, Leone M (2015) Norepinephrine: not too much, too long. Shock 44:305–309
Vincent JL, Nielsen ND, Shapiro NI, Gerbasi ME, Grossman A, Doroff R, Zeng F, Young PJ, Russell JA (2018) Mean arterial pressure and mortality in patients with distributive shock: a retrospective analysis of the MIMIC-III database. Ann Intensive Care 8:107
Lamontagne F, Day AG, Meade MO, Cook DJ, Guyatt GH, Hylands M, Radermacher P, Chretien JM, Beaudoin N, Hebert P, D’Aragon F, Meziani F, Asfar P (2018) Pooled analysis of higher versus lower blood pressure targets for vasopressor therapy septic and vasodilatory shock. Intensive Care Med 44:12–21
Levy MM (2014) Early goal-directed therapy: what do we do now? Crit Care (Lond Engl) 18:705
Rajaram SS, Desai NK, Kalra A, Gajera M, Cavanaugh SK, Brampton W, Young D, Harvey S, Rowan K (2013) Pulmonary artery catheters for adult patients in intensive care. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD003408.pub3
Peake SL, Delaney A, Bailey M, Bellomo R, Cameron PA, Cooper DJ, Higgins AM, Holdgate A, Howe BD, Webb SA, Williams P (2014) Goal-directed resuscitation for patients with early septic shock. N Engl J Med 371:1496–1506
Mouncey PR, Osborn TM, Power GS, Harrison DA, Sadique MZ, Grieve RD, Jahan R, Harvey SE, Bell D, Bion JF, Coats TJ, Singer M, Young JD, Rowan KM (2015) Trial of early, goal-directed resuscitation for septic shock. N Engl J Med 372:1301–1311
Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, Pike F, Terndrup T, Wang HE, Hou PC, LoVecchio F, Filbin MR, Shapiro NI, Angus DC (2014) A randomized trial of protocol-based care for early septic shock. N Engl J Med 370:1683–1693
Feng M, McSparron JI, Kien DT, Stone DJ, Roberts DH, Schwartzstein RM, Vieillard-Baron A, Celi LA (2018) Transthoracic echocardiography and mortality in sepsis: analysis of the MIMIC-III database. Intensive Care Med 44:884–892
Vignon P, Repesse X, Begot E, Leger J, Jacob C, Bouferrache K, Slama M, Prat G, Vieillard-Baron A (2017) Comparison of echocardiographic indices used to predict fluid responsiveness in ventilated patients. Am J Respir Crit Care Med 195:1022–1032
Vignon P, Begot E, Mari A, Silva S, Chimot L, Delour P, Vargas F, Filloux B, Vandroux D, Jabot J, François B, Pichon N, Clavel M, Levy B, Slama M, Riu-Poulenc B (2018) Hemodynamic assessment of patients with septic shock using transpulmonary thermodilution and critical care echocardiography: a comparative study. Chest 153:55–64
Singh K, Mayo P (2018) Critical care echocardiography and outcomes in the critically ill. Curr Opin Crit Care 24:316–321
Vieillard-Baron A, Slama M, Mayo P, Charron C, Amiel JB, Esterez C, Leleu F, Repesse X, Vignon P (2013) A pilot study on safety and clinical utility of a single-use 72-hour indwelling transesophageal echocardiography probe. Intensive Care Med 39(4):629–635
Cioccari L, Zante B, Bloch A, Berger D, Limacher A, Jakob SM, Takala J, Merz TM (2018) Effects of hemodynamic monitoring using a single-use transesophageal echocardiography probe in critically ill patients—study protocol for a randomized controlled trial. Trials 19:362
Cioccari L, Zante B, Bloch A, Berger D, Limacher A, Jakob SM, Takala J, Merz TM (2018) Correction to: effects of hemodynamic monitoring using a single-use transesophageal echocardiography probe in critically ill patients—study protocol for a randomized controlled trial. Trials 19:587
Ait-Oufella H, Bige N, Boelle PY, Pichereau C, Alves M, Bertinchamp R, Baudel JL, Galbois A, Maury E, Guidet B (2014) Capillary refill time exploration during septic shock. Intensive Care Med 40:958–964
Varis E, Pettila V, Poukkanen M, Jakob SM, Karlsson S, Perner A, Takala J, Wilkman E, Group FS (2017) Evolution of blood lactate and 90-day mortality in septic shock. A post hoc analysis of the FINNAKI study. Shock 47:574–581
Hochman JS, Sleeper LA, Godfrey E, McKinlay SM, Sanborn T, Col J, LeJemtel T (1999) SHould we emergently revascularize occluded coronaries for cardiogenic shock: an international randomized trial of emergency PTCA/CABG-trial design. The SHOCK Trial Study Group. Am Heart J 137:313–321
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC (2016) The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 315:801–810
Doig GS, Simpson F (2005) Randomization and allocation concealment: a practical guide for researchers. J Crit Care 20:187–191 (discussion 191–183)
Cioccari L, Baur HR, Berger D, Wiegand J, Takala J, Merz TM (2013) Hemodynamic assessment of critically ill patients using a miniaturized transesophageal echocardiography probe. Crit Care (Lond Engl) 17:R121
Fine JP, Gray RJ (1999) A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94:496–509
Rivers EP, Yataco AC, Jaehne AK, Gill J, Disselkamp M (2015) Oxygen extraction and perfusion markers in severe sepsis and septic shock: diagnostic, therapeutic and outcome implications. Curr Opin Crit Care 21:381–387
Boulain T, Garot D, Vignon P, Lascarrou JB, Desachy A, Botoc V, Follin A, Frat JP, Bellec F, Quenot JP, Mathonnet A, Dequin PF (2014) Prevalence of low central venous oxygen saturation in the first hours of intensive care unit admission and associated mortality in septic shock patients: a prospective multicentre study. Crit Care (Lond Engl) 18:609
Cecconi M, De Backer D, Antonelli M, Beale R, Bakker J, Hofer C, Jaeschke R, Mebazaa A, Pinsky MR, Teboul JL, Vincent JL, Rhodes A (2014) Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med 40:1795–1815
Harvey S, Harrison DA, Singer M, Ashcroft J, Jones CM, Elbourne D, Brampton W, Williams D, Young D, Rowan K (2005) Assessment of the clinical effectiveness of pulmonary artery catheters in management of patients in intensive care (PAC-Man): a randomised controlled trial. Lancet (Lond Engl) 366:472–477
Takala J, Ruokonen E, Tenhunen JJ, Parviainen I, Jakob SM (2011) Early non-invasive cardiac output monitoring in hemodynamically unstable intensive care patients: a multi-center randomized controlled trial. Crit Care (Lond Engl) 15:R148
Wheeler AP, Bernard GR, Thompson BT, Schoenfeld D, Wiedemann HP, deBoisblanc B, Connors AF Jr, Hite RD, Harabin AL (2006) Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med 354:2213–2224
Funding
ImaCor Inc, Garden City, NY, USA, provided 100 hTEE probes free of charge for the study. The funder had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Dr Takala has, after his retirement from the Department of Intensive Care Medicine in August 2018, provided paid consultancy services for the Medical Director and the Director of Technology and Innovation of the Inselspital, Bern University Hospital, and to Nestec SA. The Department of Intensive Care Medicine has, or has had in the past, research contracts with Orion Corporation, Abbott Nutrition International, B. Braun Medical AG, CSEM SA, Edwards Lifesciences Services GmbH, Kenta Biotech Ltd, Maquet Critical Care AB, Omnicare Clinical Research AG and research & development/consulting contracts with Edwards Lifesciences SA, Maquet Critical Care AB, and Nestlé. The money was paid into a departmental fund, and nine of the authors received financial gain. The Department of Intensive Care Medicine has received unrestricted educational grants from the following organizations for organizing a quarterly postgraduate educational Symposium, the Berner Forum for Intensive Care (until 2015): Fresenius Kabi, GSK, MSD, Lilly, Baxter, Astellas, AstraZeneca, B | Braun, CSL Behring, Maquet, Novartis, Covidien, Nycomed, Pierre Fabre Pharma AG (formerly known as RobaPharm), Pfizer, Orion Pharma, Bard Medica S.A., Abbott AG, Anandic Medical Systems. The Department of Intensive Care Medicine has received unrestricted educational grants from the following organizations for organizing bi-annual postgraduate courses in the fields of critical care ultrasound, management of ECMO and mechanical ventilation: Pierre Fabre Pharma AG (formerly known as RobaPharm), Pfizer AG, Bard Medica S.A., Abbott AG, Anandic Medical Systems, PanGas AG Healthcare, Orion Pharma, Bracco, Edwards Lifesciences AG, Hamilton Medical AG, Fresenius Kabi (Schweiz) AG, Getinge Group Maquet AG, Dräger Schweiz AG, Teleflex Medical GmbH.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Merz, T.M., Cioccari, L., Frey, P.M. et al. Continual hemodynamic monitoring with a single-use transesophageal echocardiography probe in critically ill patients with shock: a randomized controlled clinical trial. Intensive Care Med 45, 1093–1102 (2019). https://doi.org/10.1007/s00134-019-05670-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-019-05670-6