Abstract
Introduction
More than 20 years have passed since we reported our results of treating patients with the acute respiratory distress syndrome (ARDS) with inhaled nitric oxide (iNO). The main finding was that iNO alleviated pulmonary hypertension (PH) by selective vasodilation of pulmonary vessels in ventilated lung areas. This, in turn, improved arterial oxygenation.
Methods
We now set out to review the time span between the discovery of NO in 1987 and today in order to identify and describe interesting areas of research and clinical practice surrounding the application of iNO.
Major Findings
Enhancement of ventilation–perfusion matching and alleviation of PH in ARDS, treatment of PH of the newborn, and treatment of perioperative PH in congenital heart disease serve as just a few exciting examples for the successful use of iNO. Breathing NO prevents PH induced by stored blood transfusions or sickle cell disease. Exploiting the anti-inflammatory properties of NO helps to treat malaria.
Discussion
Regarding the use of iNO in ARDS, there remains the unresolved question of whether important outcome parameters can be positively influenced. At first glance, several randomized controlled trials and meta-analyses seem to send the clear message: “There is none!” Careful analyses, however, leave sufficient room for doubt that the ideal study to produce the unequivocal proof for the inability of iNO to positively impact on important outcome parameters has, as yet, not been conducted.
Conclusion
In summary, the discovery of and research on the many positive effects of iNO has improved care of critically ill patients worldwide. It is a noble effort to continue on this path.
Similar content being viewed by others
Inhaled nitric oxide
In 1993, we published a paper in the New England Journal of Medicine reporting 10 patients with severe acute respiratory distress syndrome (ARDS) who inhaled 5–20 parts per million (ppm by volume) nitric oxide (NO). Breathing NO caused rapid and selective vasodilation of pulmonary vessels in ventilated lung regions leading to increased arterial oxygenation [1].
Historical context
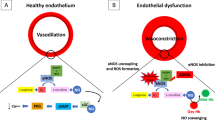
Pulmonary arterial hypertension (PAH) and intrapulmonary right-to-left shunting of venous blood are key and universal characteristics of ARDS. PAH contributes to lung oedema and, when PAH is severe, it can produce right heart dysfunction and cardiac failure. Reducing the elevated pulmonary artery pressure (PAP) was assumed to be beneficial. Before 1993, the drugs studied to reduce the PAP in ARDS were intravenous vasodilators, which reduced both the PAP and also the systemic blood pressure. Since patients with ARDS often have systemic vasodilation resulting from concomitant sepsis, intravenous vasodilators further decreased the systemic vascular resistance and arterial pressure, and at times produced right ventricular ischemia and consequent heart failure. Moreover, infusing an intravenous vasodilator produced global vasodilation of all the vessels. During ARDS, systemic vasodilator therapy indiscriminately vasodilated the pulmonary vasculature in both ventilated as well as non-ventilated lung regions, increasing ventilation–perfusion mismatch and leading to deterioration of pulmonary gas exchange. Therefore, in most clinical settings with acute lung injury, it was not feasible or useful to infuse intravenous vasodilators to treat PAH. Beginning in 1980, Furchgott et al. [2] reported that acetylcholine stimulates endothelial cell receptors triggering the production of a substance that diffuses into vascular smooth muscle cells and causes relaxation. He named this substance “endothelium-derived relaxing factor” (EDRF). In 1987, two independent researchers established that EDRF was the small gaseous molecule NO [3, 4]. Since NO is a byproduct of producing various gases and a common atmospheric pollutant, it was readily available to laboratory researchers. Within a short period, studies in large animals and humans demonstrated that, when NO was inhaled in low doses (5–80 ppm), it rapidly produced selective pulmonary vasodilation (Fig. 1). NO is instantly inactivated by the dioxygenation reaction when it diffuses into blood vessels containing oxyhaemoglobin. In 1991, Frostell et al. [5] reported the ability of inhaled NO to selectively induce pulmonary vasodilation in an ovine model of PAH. Pepke-Zaba et al. [6] studied the effect of inhaled NO in adults that suffered from chronic PAH. These and other promising clinical studies rapidly led us to evaluate inhaled NO as a therapy in patients with severe ARDS. Figure 2 displays selected temporal highlights surrounding the discovery of inhaled NO as a selective pulmonary vasodilator.
Our paper in the historical context, highlights of iNO-history, and therapeutic use of iNO in adults. EDRF endothelium-derived relaxing factor, NO nitric oxide, iNO inhaled nitric oxide, ARDS acute respiratory distress syndrome, RVEF right ventricular ejection fraction, PAH pulmonary arterial hypertension, PRBC packed red blood cells, PaO 2 partial pressure of oxygen in arterial blood, numbers in brackets refer to the reference list
Methods and major findings of our study
In our study, we compared the short-term effects of breathing two concentrations of NO (18 and 36 ppm) with an intravenous infusion of prostacyclin (4 ng/kg/min). We measured systemic and pulmonary haemodynamics, pulmonary gas exchange and ventilation–perfusion distributions (assessed by the multiple inert gas elimination technique [7, 8]). Each drug was given for 40 min to ten consecutive patients with severe ARDS. In these patients, we found breathing NO induced selective pulmonary vasodilation leading to an immediate reduction of PAP. NO inhalation did not alter either the systemic arterial pressure (SAP) or cardiac output. Surprisingly, during NO breathing, we recorded a concurrent improvement of arterial oxygenation. Analysis of ventilation–perfusion distributions revealed that this effect was caused by shifting pulmonary blood flow from collapsed and shunting lung areas to ventilated regions. In contrast, intravenous prostacyclin reduced PAP, SAP and systemic oxygenation assessed as the ratio of the partial pressure of arterial oxygen divided by the fraction of inspired oxygen (PaO2/FiO2).
In seven of ten patients, NO inhalation was continued for periods of 3–53 days. NO treatment was terminated if the PaO2/FiO2 ratio remained >250 mmHg in patients without extracorporeal membrane oxygenation (ECMO) during daily periods of NO cessation. The daily interruption of NO inhalation for 30 min revealed that breathing NO caused a reproducible decrease in PAP without influencing mean SAP and cardiac output, as well as augmenting arterial oxygenation due to a decrease of pulmonary right-to-left shunting (Fig. 3). Our study was the first to show that, in patients with ARDS, the inhalation of a gaseous vasodilator (NO) induced selective vasodilation in ventilated lung regions, a concept which was later confirmed by other researchers who studied inhaled NO or aerosolized prostacyclin [9].
Haemodynamic function and gas exchange before, during, and after brief interruptions (arrows) of nitric oxide inhalation (bars) during the first 6 days of treatment in seven patients with ARDS. Values are means ± SE (solid symbols); also shown (open symbols) are the means ± SE of the individual differences between the values for the effect of treatment and the means of the values determined before and after interruption of nitric oxide therapy. The standard errors for the treatment effects were small, indicating that the effects of withdrawal of nitric oxide were clear and precisely estimated. Each asterisk denotes a significant difference from the means of the values determined before and after interruption of nitric oxide therapy (from [1], Copyright © (1993) Massachusetts Medical Society. Reprinted with permission)
Long-term effects of inhaled NO in ARDS
In a subsequent study, we reported that the use of a selective pulmonary vasodilator improved the ejection fraction of the right heart without producing systemic vasodilation [10]. Due to the short-term haemodynamic and gas exchange focus of our initial study of ten ARDS patients, we could not learn whether inhaled NO could improve important ARDS outcome parameters like organ failure-free days or survival. Subsequently, nine randomized controlled trials (RCT) have been performed to learn the long-term effects of breathing NO during ARDS [11–19]. These studies again demonstrated that inhaled NO reduced PAP and intrapulmonary right-to-left shunt and improved arterial oxygenation. These favourable effects, however, only appeared to last for 24–48 h. In ARDS patients, there were no significant differences of organ failure-free days, length of ICU stay or hospital mortality with inhaled NO.
A major criticism of these nine RCTs is that they were performed in patients with both severe ARDS (PaO2/FiO2 <100 mmHg) and moderate ARDS (PaO2/FiO2 >100 and <200 mmHg). A recent meta-analysis analysed all nine studies (including 1,142 patients) with respect to the effect of breathing NO upon mortality, and performed a sub-analysis of seven studies (1,070 patients total) to attempt to differentiate between the effect of NO treatment in patients with moderate and severe ARDS [20]. No beneficial effect of inhaled NO with respect to long-term outcome was discerned in either severity group. One limitation of this study is that the authors included only 329 patients in their analysis of severe ARDS and no trend was detectable. In all of these studies patients usually received a tidal volume of over 6 ml/kg ideal body weight during mechanical ventilation. Up to now, it is unknown, but conceivable, that a protective ventilator strategy in combination with inhaled NO leads to more positive results. Experience from the use of prone position in ARDS has taught us that an improvement in outcome was pronounced if this treatment option was combined with a lung protective mechanical ventilation strategy [21].
Additive or synergistic effects of inhaled nitric oxide (iNO) and other treatment modules were reported by a number of other studies. These studies detected enhanced beneficial effects when iNO was combined with measures to enhance alveolar recruitment, such as positive end-expiratory pressure [22], high frequency ventilation [23], recruitment manoeuvres [18], prone position [24], and surfactant instillation [25, 26]. There is, however, no randomized trial assessing such combined effect on the outcome of patients with severe ARDS.
It is possible that inhaled NO might reduce the need for more invasive treatment strategies such as ECMO. This would be of interest, as Gerlach et al. [17] demonstrated, that the inhaled NO treatment group had a reduced need for invasive and costly extracorporeal membrane oxygenation (ECMO) therapy.
In summary, in the current era of protective mechanical ventilation, some clinicians employ NO breathing for a period of 24–48 h as a rescue intervention for severely hypoxemic patients to buy time and allow other therapies (ECMO, prone positioning, etc.) to improve the patients’ pulmonary gas exchange. To date, however, there remain doubts whether improved oxygenation is an adequate outcome parameter in ARDS patients [27–30]. Furthermore, iNO-response may differ dose- and time-dependently between patients [31–33]. About one-third of all ARDS patients are non-responders to iNO, i.e. their oxygenation does not improve relevantly. It is disappointing, however, that, regardless of whether patients respond or do not respond, iNO does not influence important long-term outcome parameters [14].
Inhaled NO in neonatal hypoxaemia and persistent pulmonary hypertension
Term gestation PPH
While we were studying inhaled NO in adult ARDS, breathing NO was evaluated in term gestation neonates with persistent pulmonary hypertension (PPH) and other hypoxaemic preterm infants. PPH in the newborn may occur as a consequence of various congenital and acquired conditions: PPH may be idiopathic or associated with premature closure of the ductus arteriosus, meconium aspiration, prematurity, or lung hypoplasia. In cases of premature closure of the ductus arteriosus and closure of the foramen ovale, right heart failure and systemic hypotension can occur. If PPH co-exists with a patent ductus arteriosus and foramen ovale, right-to-left shunting of venous blood can produce severe systemic hypoxaemia. In 1992, two independent teams of researchers described the beneficial effects of inhaled NO in PPH by reducing the PAP and right-to-left shunting of venous blood which markedly improved systemic oxygenation [34, 35]. Reduction of pulmonary hypertension occurred without decreasing the systemic blood pressure. Several years later, two prospective RCTs demonstrated breathing NO by PPHN infants reduced the need for invasive and increased survival rates (as a combined endpoint). In addition, neonates treated with NO had better lung function at 30 days [36, 37]. In 2001, these studies and others led the FDA to approve inhaled NO for treating hypoxemia in term neonates.
Preterm infants
Breathing NO has been studied in preterm infants. Up to the present, 14 prospective RCTs have been carried out in preterm infants to learn whether NO treatment can provide benefits to preterm infants [38–51].
Some of these preterm infant trials reported a benefit of inhaled NO, but some did not. This might have been due to their heterogeneity in design [including differing dosage of NO, timing of intervention, indication (hypoxemia vs. chronic lung disease), patient population, intervention time and duration of treatment]. In 2010, a Cochrane review analysed these 14 RCTs in pre-term infants and did not find significant differences of mortality rates or the development of chronic lung disease [52]. A consecutive meta-analysis of 12 of these 14 trials in preterm infants based on an individual patient data reanalysis did not reveal statistically significant effects of breathing NO on mortality rates, the development of chronic lung disease {59 vs. 61 %: relative risk (RR): 0.96 [95 % confidence interval (CI): 0.92–1.01]; P = 0.11}, or the incidence of severe neurologic events diagnosed by imaging [25 vs. 23 %: RR: 1.12 (95 % CI: 0.98–1.28); P = 0.09] [53].
Inhaled NO in cardiac surgery
Paediatric cardiac surgery
Cardiac surgery, especially when repairing congenital heart disease, is often complicated by perioperative pulmonary hypertension. In cases of pulmonary hypertension following surgery for congenital heart disease, several case series have reported that inhalation of NO selectively reduced the pulmonary vascular resistance and improved the patients’ condition [54–56]. Nevertheless, a review that analysed the four published randomized and quasi-randomized controlled trials comparing NO breathing with placebo or conventional management [57–60], did not reveal any benefit with respect to survival or haemodynamics [61]. A recently published review that analysed 15 clinical trials (including case reports, pilot studies, prospective and retrospective investigations, RCT, and cross-over studies) concluded that, in paediatric patients with pulmonary hypertension after surgery for congenital heart disease, inhaled NO effectively improved haemodynamics and pulmonary gas exchange [62].
Adult cardiac surgery
NO breathing can be used pre- and postoperatively to test pulmonary vasoreactivity in paediatric and adult patients with PAH [63]. Vasodilator testing results can help determine the surgical strategy in heart and/or lung transplantation [64]. In the period following heart transplantation complicated by life-threatening pulmonary hypertension and consecutive right heart failure, breathing NO reduced pulmonary hypertension and improved right heart function [65]. It has been impossible to randomize patients receiving left ventricular assist devices to study the effects of breathing NO, since cardiac surgeons are past the point of equipoise and refuse to randomize their patients, since adding a right ventricular assist device for PAH with RV failure would be an excessive additional risk for the patient.
Side effects and toxicology of inhaled NO
Centuries ago, Philippus Theophrastus Bombastus of Hohenheim, better known as Paracelsus (1493–1541), was a congenial physician, astrologer, and theologian who enriched the art of healing by his deep insights into the nature of diseases. The following quotation is traced back to Paracelsus: “All substances are poisons; there is none which is not. The right dose differentiates a poison from a remedy”. This phrase is taken to heart by each physician who prescribes inhaled NO as the effective dose and the harmful dose of NO lie close together.
NO is a free radical that oxidizes in the presence of oxygen (O2) to form the highly toxic gas, nitrogen dioxide (NO2). When NO2 dissolves in water, it produces nitric acid. NO2 levels of 2 ppm or more may increase alveolar permeability, whereas breathing concentrations of NO2 above 10 ppm can produce severe lung damage. The Cochrane Collaboration analysed 14 RCT on the use of iNO in patients with acute lung injury or ARDS and found no increased risk for NO2 formation [66].
Methaemoglobin forms when NO binds to oxyhaemoglobin in erythrocytes, but the ferric haemoglobin is rapidly reduced to ferrous haemoglobin by methaemoglobin reductase. Small amounts of methaemoglobin can be detected in patients who breathe high NO concentrations (e.g. 80 ppm); however, severe methaemoglobinaemia in conjunction with iNO-therapy is extremely rare [66, 67].
Coagulation alterations have been reported in some patients treated with inhaled NO, but a systematic review by the Cochrane Collaboration revealed no greater risk of bleeding problems in NO-treated patients with ARDS [66]. Nephrotoxicity of iNO is another possible problem of patients with acute lung injury and ARDS. Two large meta-analyses confirmed a small risk of renal impairment when ARDS patients are treated with NO [66, 67]. However, as ARDS patients are often septic, they have a major independent risk of renal failure. Since neonates given NO for PPHN do not have an increased incidence of renal failure, it is unlikely that breathing NO is toxic to the kidney.
Weaning the patient from breathing NO confronts the clinician with a special management problem: a “rebound phenomenon” of pulmonary vasoconstriction following acute NO withdrawal. Especially, when NO treatment is abruptly ceased, acute rebound vasoconstriction is common. Worsening of gas exchange was found in 48 % of acutely weaned patients and 26 % exhibited haemodynamic impairment [68]. Thus, NO breathing must be weaned slowly and judiciously.
In summary, NO breathing can be managed safely, given adequate attention to the well-described side effects (Table 1).
Future directions in inhaled NO application
Malaria
Two recent laboratory studies have reported that NO may play a useful role as an adjunctive therapy to artesunate in severe or cerebral malaria due to its anti-inflammatory properties [69, 70]. Since plasma and urine nitrate levels are extremely low in severe malaria patients there may be value in replacing NO biometabolites with inhaled NO. In malaria, angiopoetin-2 release contributes to activation of the endothelium, which can be inhibited by NO. NO decreases inflammation and reduces the adhesion of parasitized erythrocytes to endothelium [69]. Thus inhaled NO could reduce inflammation and buy time for therapeutic drugs (e.g., artesunate) to eliminate the malaria parasites [70]. At present, there are two ongoing phase II RCTs in Uganda studying the safety and efficacy of breathing 80 ppm NO versus N2, both gases given with artesunate, with a primary endpoint of altering angiopoietin levels [71, 72]. The latter is a quantitative biomarker of malaria severity [73, 74]. Unfortunately, recent abstracts suggest that, although breathing NO was safe to administer in severe and cerebral malaria, there was no evidence of effectiveness by altering angiopoietin levels (personal communication from Ryan Caroll and Kevin Kain).
Stored blood transfusion
Transfusion of packed red blood cells stored for over 2 weeks is an independent risk factor for organ failure and mortality [75]. Recently, it was reported that resuscitation of lambs from haemorrhagic shock with autologous stored red blood cells induces pulmonary hypertension and inflammation. Breathing NO prevented these adverse consequences [76]. Transfusion of stored leukoreduced autologous red cells produces transient acute pulmonary hypertension in obese human volunteers with endothelial dysfunction. Breathing NO prevents the pulmonary hypertension. This is an area of active investigation.
INO in sickle cell disease
Despite initial enthusiasm for treating sickle cell crisis patients with inhaled NO, a large multicentre study showed no reduction of pain scores with NO breathing [77]. Thus, there is no reason to treat painful crises with inhaled NO. However, the pulmonary hypertension that occurs in sickle cell chest crisis does respond by vasodilation to inhaled NO [78].
Conclusion
The study we published in 1993 was one of the first to provide evidence for selective pulmonary vasodilation produced by breathing NO in adult ARDS patients. It offered the remarkable advantage of lowering the elevated pulmonary artery pressures, improving arterial oxygenation, and protecting the right ventricle from failure without reducing systemic arterial resistance or pressure. iNO proved superior to classical intravenous vasodilators in ARDS, since breathing NO reduced and did not increase the pulmonary right-to-left shunt fraction. Researchers and clinicians working in the fields of neonatology and cardiac surgery were stimulated by this paper to explore NO therapy for hypoxemic infants with PPH, and children and adults undergoing cardiac surgery. Unfortunately, researchers studying ARDS never demonstrated an improved outcome with iNO treatment. However, breathing NO is still used to buy time to institute other therapies in severely hypoxemic ARDS. Perhaps, now that low volume ventilation has become standard therapy in ARDS, a trial of breathing NO to improve the outcome of ARDS patients should be considered.
Future research may yield synergistic beneficial effects in terms of outcome between iNO and other advanced ARDS treatment modules.
References
Rossaint R, Falke KJ, López F, Slama K, Pison U, Zapol WM (1993) Inhaled nitric oxide for the adult respiratory distress syndrome. N Engl J Med 328:399–405
Furchgott RF, Zawadzki JV (1980) The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288:373–376
Palmer RM, Ferrige AG, Moncada S (1987) Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 327:524–526
Ignarro LJ, Byrns RE, Buga GM, Woods KS (1987) Endothelium derived relaxing factor from pulmonary artery and vein possesses pharmacological and chemical properties that are identical to those of nitric oxide radical. Circ Res 61:866–879
Frostell C, Fratacci MD, Wain JC, Jones R, Zapol WM (1991) Inhaled nitric oxide: a selective pulmonary vasodilator reversing hypoxic pulmonary vasoconstriction. Circulation 83:2038–2047
Pepke-Zaba J, Higenbottam TW, Dinh-Xuan AT, Stone D, Wallwork J (1991) Inhaled nitric oxide as a cause of selective pulmonary vasodilatation in pulmonary hypertension. Lancet 338:1173–1174
Wagner PD, Saltzman HA, West JB (1974) Measurement of continuous distributions of ventilation–perfusion ratios: theory. J Appl Physiol 36:588–599
Evans JW, Wagner PD (1977) Limits on VA/Q distributions from analysis of experimental inert gas elimination. J Appl Physiol Respir Environ Exerc Physiol 42:889–898
Pappert D, Busch T, Gerlach H, Lewandowski K, Radermacher P, Rossaint R (1995) Aerosolized prostacyclin versus inhaled nitric oxide in children with severe acute respiratory distress syndrome. Anesthesiology 82:1507–1511
Rossaint R, Slama K, Steudel W, Gerlach H, Pappert D, Veit S, Falke K (1995) Effect of inhaled nitric oxide on right ventricular function in severe acute respiratory distress syndrome. Intensive Care Med 21:197–203
Dellinger RP, Zimmerman JL, Taylor RW, Straube RC, Hauser DL, Criner GJ, Davis K, Hyers TM, Papadakos P (1998) Effects of inhaled nitric oxide in patients with acute respiratory distress syndrome: results of a randomized phase II trial. Inhaled nitric oxide in ARDS study group. Crit Care Med 26:15–23
Michael JR, Barton RG, Saffle JR, Mone M, Markewitz BA, Hillier K, Elstad MR, Campbell EJ, Troyer BE, Whatley RE, Liou TG, Samuelson WM, Carveth HJ, Hinson DM, Morris SE, Davis BL, Day RW (1998) Inhaled nitric oxide versus conventional therapy: effect on oxygenation in ARDS. Am J Respir Crit Care Med 157:1372–1380
Troncy E, Collet JP, Shapiro S, Guimond JG, Blair L, Ducruet T, Francoeur M, Charbonneau M, Blaise G (1998) Inhaled nitric oxide in acute respiratory distress syndrome: a pilot randomized controlled study. Am J Respir Crit Care Med 157:1483–1488
Lundin S, Mang H, Smithies M, Stenqvist O, Frostell C (1999) Inhalation of nitric oxide in acute lung injury: results of a European multicentre study. The European Study Group of Inhaled Nitric Oxide. Intensive Care Med 25:911–919
Payen D, Vallet B, Group d’etude du NO dans l’ARDS (1999) Results of the French prospective multicentric randomized double-blind placebo-controlled trial on inhaled nitric oxide (NO) in ARDS [abstract]. Intensive Care Med 25(Suppl 1):S166
Mehta S, Simms HH, Levy MM, Hill NS, Schwartz W, Nelson D, Short K, Klinger JR (2001) Inhaled nitric oxide improves oxygenation acutely but not chronically in acute respiratory distress syndrome: a randomized, controlled trial. J Appl Res 1:73–84
Gerlach H, Keh D, Semmerow A, Busch T, Lewandowski K, Pappert DM, Rossaint R, Falke KJ (2003) Dose-response characteristics during long-term inhalation of nitric oxide in patients with severe acute respiratory distress syndrome: a prospective, randomized, controlled study. Am J Respir Crit Care Med 167:1008–1015
Park KJ, Lee YJ, Oh YJ, Lee KS, Sheen SS, Hwang SC (2003) Combined effects of inhaled nitric oxide and a recruitment maneuver in patients with acute respiratory distress syndrome. Yonsei Med J 44:219–226
Taylor RW, Zimmerman JL, Dellinger RP, Straube RC, Criner GJ, Davis K Jr, Kelly KM, Smith TC, Small RJ, Inhaled Nitric Oxide in ARDS Study Group (2004) Inhaled Nitric Oxide in ARDS Study Group: low-dose inhaled nitric oxide in patients with acute lung injury: a randomized controlled trial. JAMA 291:1603–1609
Adhikari NKJ, Dellinger RP, Lundin S, Payen D, Vallet B, Gerlach H, Park KJ, Mehta S, Slutsky AS, Friedrich JO (2014) Inhaled nitric oxide does not reduce mortality in patients with acute respiratory distress syndrome regardless of severity: systematic review and meta-analysis. Crit Care Med 42:404–412
Lee JM, Bae W, Lee YJ, Cho YJ (2014) The efficacy and safety of prone positional ventilation in acute respiratory distress syndrome: updated study-level meta-analysis of 11 randomized controlled trials. Crit Care Med 42:1252–1262
Okamoto K, Kukita I, Hamaguchi M, Motoyama T, Muranaka H, Harada T (2000) Combined effects of inhaled nitric oxide and positive end-expiratory pressure during mechanical ventilation in acute respiratory distress syndrome. Artif Organs 24:390–395
Hoehn T, Krause MF (1998) Synergistic effects of high-frequency ventilation and inhaled nitric oxide in the treatment of hypoxemic respiratory failure in infancy. Pediatr Pulmonol 26:228–230
Johannigman JA, Davis K, Miller SL, Campbell RS, Luchette FA, Frame SB, Branson RD (2001) Prone positioning and inhaled nitric oxide: synergistic therapies for acute respiratory distress syndrome. J Trauma 50:589–595
Hartog A, Gommers D, van’t Veen A, Erdmann W, Lachmann B (1997) Exogenous surfactant and nitric oxide have a synergistic effect in improving gas exchange in experimental ARDS. Adv Exp Med Biol 428:277–279
Zhu GF, Su B, Niu SF, Cai YY, Lin K, Lindwall R, Robertson B (1998) Combined surfactant therapy and inhaled nitric oxide in rabbits with oleic-acid induced acute respiratory syndrome. Am J Respir Crit Care Med 158:437–443
Net ARDS (2000) Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 342:1301–1308
Cheifetz IM, Hamel DS (2006) Is permissive hypoxemia a beneficial strategy for pediatric acute lung injury? Respir Care Clin N Am 12:359–369
Gilbert-Kawai ET, Mitchell K, Martin D, Carlisle J, Grocott MP (2014) Permissive hypoxaemia versus normoxaemia for mechanically ventilated critically ill patients. Cochrane Database Syst Rev 7(5):CD009931
MacIntyre NR (2013) Supporting oxygenation in acute respiratory failure. Respir Care 58:142–148
Bigatello LM, Hurford WE, Kacmarek RM, Roberts JDJ, Zapol WM (1994) Prolonged inhalation of low concentrations of nitric oxide in patients with severe adult respiratory distress syndrome. Effects on pulmonary hemodynamics and oxygenation. Anesthesiology 80:761–770
Gerlach H, Rossaint R, Pappert D, Falke KJ (1993) Time-course and dose response of nitric oxide inhalation for systemic oxygenation and pulmonary hypertension in patients with adult respiratory distress syndrome. Eur J Clin Invest 23:499–502
Lundin S, Westfelt UN, Stenqvist O, Frostell C (1996) Response to nitric oxide inhalation in early acute lung injury. Intensive Care Med 22:728–734
Roberts JD, Polaner DM, Lang P, Zapol WM (1992) Inhaled nitric oxide in persistent pulmonary hypertension of the newborn. Lancet 340:818–819
Kinsella JP, Neish SR, Shaffer E, Abman SH (1992) Low-dose inhalation nitric oxide in persistent pulmonary hypertension of the newborn. Lancet 340:819–820
Clark RH, Kueser TJ, Walker MW, Southgate WM, Huckaby JL, Perez JA, Roy BJ, Keszler M, Kinsella JP (2000) Low-dose nitric oxide therapy for persistent pulmonary hypertension of the newborn. Clinical Inhaled Nitric Oxide Research Group. N Engl J Med 342:469–474
Roberts JD, Fineman JR, Morin FC 3rd, Shaul PW, Rimar S, Schreiber MD, Polin RA, Zwass MS, Zayek MM, Gross I, Heymann MA, Zapol WM (1997) Inhaled nitric oxide and persistent pulmonary hypertension of the newborn. The Inhaled Nitric Oxide Study Group. N Engl J Med 336:605–610
Subhedar N, Ryan S, Shaw N (1997) Open randomized controlled trial of inhaled nitric oxide and early dexamethasone in high risk preterm infants. Arch Dis Child Fetal Neonatal Ed 77:F185–F190
The Franco-Belgium Collaborative NO Trial Group (1999) Early compared with delayed inhaled nitric oxide in moderately hypoxaemic neonates with respiratory failure: a randomized controlled trial. Lancet 354:1066–1071
Kinsella JP, Walsh WF, Bose CL, Gerstmann DR, Labella JJ, Sardesai S, Walsh-Sukys MC, McCaffrey MJ, Cornfield DN, Bhutani VK, Cutter GR, Baier M, Abman SH (1999) Inhaled nitric oxide in premature neonates with severe hypoxaemic respiratory failure: a randomized controlled trial. Lancet 354:1061–1065
Srisuparp P, Heitschmidt M, Schreiber MD (2002) Inhaled nitric oxide therapy in premature infants with mild to moderate respiratory distress syndrome. J Med Assoc Thai 85(suppl 2):S469–S478
Schreiber MD, Gin-Mestan K, Marks JD, Huo D, Lee G, Srisuparp P (2003) Inhaled nitric oxide in premature infants with the respiratory distress syndrome. N Engl J Med 349:2099–2107
Field D, Elbourne D, Truesdale A, Diallo K, INNOVO Trial Collaborating Group (2005) Neonatal ventilation with inhaled nitric oxide versus ventilatory support without inhaled nitric oxide for preterm infants with severe respiratory failure: the INNOVO multicentre randomized controlled trial. Pediatrics 115:926–936
Van Meurs K, Wright L, Ehrenkranz R, Lemons JA, Ball MB, Poole WK, Perritt R, Higgins RD, Oh W, Hudak ML, Laptook AR, Shankaran S, Finer NN, Carlo WA, Kennedy KA, Fridriksson JH, Steinhorn RH, Sokol GA, Konduri GG, Aschner JL, Stoll BJ, D’Angio CT, Stevenson DK, Preemie Inhaled Nitric Oxide Study (2005) Inhaled nitric oxide for premature infants with severe respiratory failure. N Engl J Med 353:13–22
Ballard RA, Truog WE, Cnaan A, Martin RJ, Ballard PL, Merrill JD, Walsh MC, Durand DJ, Mayock DE, Eichenwald EC, Null DR, Hudak ML, Puri AR, Golombek SG, Courtney SE, Stewart DL, Welty SE, Phibbs RH, Hibbs AM, Luan X, Wadlinger SR, Asselin JM, Coburn CE, NO CLD Study Group (2006) Inhaled nitric oxide in preterm infants undergoing mechanical ventilation. N Engl J Med 355:343–353
Dani C, Bertini G, Pezzati M, Filippi L, Cecchi A, Rubaltelli FF (2006) Inhaled nitric oxide in very preterm infants with severe respiratory distress syndrome. Acta Paediatr 95:1116–1123
Hascoet JM, Fresson J, Claris O, Hamon I, Lombet J, Liska A, Cantagrel S, Al Hosri J, Thiriez G, Valdes V, Vittu G, Egreteau L, Henrot A, Buchweiller MC, Onody P (2005) The safety and efficacy of nitric oxide therapy in premature infants. J Pediatr 146:318–323
Kinsella JP, Cutter GR, Walsh WF, Gerstmann DR, Bose CL, Hart C, Sekar KC, Auten RL, Bhutani VK, Gerdes JS, George TN, Southgate WM, Carriedo H, Couser RJ, Mammel MC, Hall DC, Pappagallo M, Sardesai S, Strain JD, Baier M, Abman SH (2006) Early inhaled nitric oxide therapy in premature newborns with respiratory failure. N Engl J Med 355:354–364
Van Meurs KP, Hintz SR, Ehrenkranz RA, Lemons JA, Ball MB, Poole WK, Perritt R, Das A, Higgins RD, Stevenson DK (2007) Inhaled nitric oxide in infants <1500 g and <34 weeks gestation with severe respiratory failure. J Perinatol 27:347–352
Su PH, Chen JY (2008) Inhaled nitric oxide in the management of preterm infants with severe respiratory failure. J Perinatol 28:112–116
Mercier JC, Hummler H, Durrmeyer X, Sanchez-Luna M, Carnielli V, Field D, Greenough A, Van Overmeire B, Jonsson B, Hallman M, Baldassarre J, EUNO Study Group (2010) Inhaled nitric oxide for prevention of bronchopulmonary dysplasia in premature babies (EUNO): a randomised controlled trial. Lancet 376:346–354
Barrington KJ, Finer N (2010) Inhaled nitric oxide for respiratory failure in preterm infants. Cochrane Database Syst Rev 8(12):CD000509
Askie LM, Ballard RA, Cutter GR, Dani C, Elbourne D, Field D (2011) Inhaled nitric oxide in preterm infants: an individual-patient data meta-analysis of randomized trials. Pediatrics 128:729–739
Journois D, Pouard P, Mauriat P, Malhere T, Vouhe P, Safran D (1994) Inhaled nitric oxide as a therapy for pulmonary hypertension after operations for congenital heart disease. J Thorac Cardiovasc 107:1129–1135
Miller OI, Celermajer DS, Deanfield JE, Macrae DJ (1994) Very low-dose inhaled nitric oxide: a selective pulmonary vasodilator after operations for congenital heart disease. J Thorac Cardiovasc Surg 108:487–494
Beghetti M, Habre W, Berner M (1995) Continuous low dose inhaled nitric oxide for treatment of severe pulmonary hypertension after cardiac surgery in paediatric patients. Br Heart J 73:65–68
Russell IA, Zwass MS, Fineman JR, Balea M, Rouine-Rapp K, Brook M, Hanley FL, Silverman NH, Cahalan MK (1998) The effects of inhaled nitric oxide on postoperative pulmonary hypertension in infants and children undergoing surgical repair of congenital heart disease. Anesth Analg 87:46–51
Miller OI, Tang SF, Keech A, Pigott NB, Beller E, Celermajer DS (2000) Inhaled nitric oxide and prevention of pulmonary hypertension after congenital heart surgery: a randomized double-blind study. Lancet 356:1464–1469
Day RW, Hawkins JA, McGough EC, Crezee KL, Orsmond GS (2000) Randomized controlled study of inhaled nitric oxide after operation for congenital heart surgery. Ann Thorac Surg 69:1907–1913
Morris K, Beghetti M, Petros A, Adatia I, Bohn D (2000) Comparison of hyperventilation and inhaled nitric oxide for pulmonary hypertension after repair of congenital heart disease. Crit Care Med 28:2974–2978
Bizzarro M, Gross I (2005) Inhaled nitric oxide for the postoperative management of pulmonary hypertension in infants and children with congenital heart disease. Cochrane Database Syst Rev 19(4):CD005055. doi:10.1002/14651858.CD005055
Checchia PA, Bronicki RA, Goldstein B (2012) Review of inhaled nitric oxide in the pediatric cardiac surgery setting. Pediatr Cardiol 33:493–505
Balzer DT, Kort HW, Day RW, Corneli HM, Kovalchin JP, Cannon BC (2002) Inhaled nitric oxide as a preoperative test (INOP Test I): the INOP Test Study Group. Circulation 106(12 Suppl 1):176–181
Haraldsson A, Kieler-Jensen N, Nathorst-Westfelt U, Bergh CH, Ricksten SE (1998) Comparison of inhaled nitric oxide and inhaled aerosolized prostacyclin in the evaluation of heart transplant candidates with elevated pulmonary vascular resistance. Chest 114:780–786
Ardehali A, Hughes K, Sadeghi A, Esmailian F, Marelli D, Moriguchi J (2001) Inhaled nitric oxide for pulmonary hypertension after heart transplantation. Transplantation 72:638–641
Afshari A, Brok J, Møller AM, Wetterslev J (2010) Inhaled nitric oxide for acute respiratory distress syndrome (ARDS) and acute lung injury in children and adults. Cochrane Database Syst Rev 7(7):CD002787
Adhikari NKJ, Burns KEA, Friedrich JO, Granton JT, Cook DJ, Meade MO (2007) Effect of nitric oxide on oxygenation and mortality in acute lung injury: systematic review and meta-analysis. BMJ 334:779–786
Christenson J, Lavoi A, O’Connor M, Bhorade S, Pohlman A, Hall JB (2000) The incidence and pathogenesis of cardiopulmonary deterioration after abrupt withdrawal of inhaled nitric oxide. Am J Respir Crit Care Med 161:1443–1449
Serirom S, Raharjo WH, Chotivanich K, Loareesuwan S, Kubes P, Ho M (2003) Anti-adhesive effect of nitric oxide on Plasmodium falciparum cytoadherence under flow. Am J Pathol 162:1651–1660
Bergmark B, Bergmark R, Beaudrap P, Boum Y, Mwanga-Amumpaire J, Ryan Carroll R, Zapol W (2012) Inhaled nitric oxide and cerebral malaria: basis of a strategy for buying time for pharmacotherapy. Pediatr Infect Dis J 31:e250–e254
Hawkes M, Opoka RO, Namasopo S, Miller C, Thorpe KE, Lavery JV, Conroy AL, Liles WC, John CC, Kain KC (2011) Inhaled nitric oxide for the adjunctive therapy of severe malaria: protocol for a randomized controlled trial. Trials 12:176
Evaluation of the efficacy and safety of inhaled nitric oxide as adjunctive treatment for cerebral malaria in children. Last updated 2013, Clinical Trials. Gov Identifier: NCT01388842
Yeo TW, Lampah DA, Gitawati R, Tjitra E, Kenangalem E, Piera K, Price RN, Duffull SB, Celermajer DS, Anstey NM (2008) Angiopoietin-2 is associated with decreased endothelial nitric oxide and poor clinical outcome in severe falciparum malaria. Proc Natl Acad Sci USA 105:17097–17102
Lovegrove FE, Tangpukdee N, Opoka RO, Lafferty EI, Rajwans N, Hawkes M, Krudsood S, Looareesuwan S, John CC, Liles WC, Kain KC (2009) Serum angiopoietin-1 and -2 levels discriminate cerebral malaria from uncomplicated malaria and predict clinical outcome in African children. PLoS ONE 4:e4912
Koch CG, Li L, Sessler DI, Figueroa P, Hoeltge GA, Mihaljevic T, Blackstone EH (2008) Duration of red-cell storage and complications after cardiac surgery. N Engl J Med 358:1229–1239
Baron DM, Beloiartsev A, Nakagawa A, Martyn T, Stowell CP, Malhotra R, Mayeur C, Bloch KD, Zapol WM (2013) Adverse effects of hemorrhagic shock resuscitation with stored blood are ameliorated by inhaled nitric oxide in lambs. Crit Care Med 41:2492–2501
Gladwin MT, Kato GJ, Weiner D, Onyekwere OC, Dampier C, Hsu L, Hagar RW, Howard T, Nuss R, Okam MM, Tremonti CK, Berman B, Villella A, Krishnamurti L, Lanzkron S, Castro O, Gordeuk VR, Coles WA, Peters-Lawrence M, Nichols J, Hall MK, Hildesheim M, Blackwelder WC, Baldassarre J, Casella JF, DeNOVO Investigators (2011) Nitric oxide for inhalation in the acute treatment of sickle cell pain crisis: a randomized controlled trial. JAMA 305:893–902
Atz AM, Wessel DL (1997) Inhaled nitric oxide in sickle cell disease with acute chest syndrome. Anesthesiology 87:988–990
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rossaint, R., Lewandowski, K. & Zapol, W.M. Our paper 20 years later: Inhaled nitric oxide for the acute respiratory distress syndrome—discovery, current understanding, and focussed targets of future applications. Intensive Care Med 40, 1649–1658 (2014). https://doi.org/10.1007/s00134-014-3458-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-014-3458-6