Abstract
Nitric oxide (NO) is a key molecule in the biology of human life. NO is involved in the physiology of organ viability and in the pathophysiology of organ dysfunction, respectively. In this narrative review, we aimed at elucidating the mechanisms behind the role of NO in the respiratory and cardio-cerebrovascular systems, in the presence of a healthy or dysfunctional endothelium. NO is a key player in maintaining multiorgan viability with adequate organ blood perfusion. We report on its physiological endogenous production and effects in the circulation and within the lungs, as well as the pathophysiological implication of its disturbances related to NO depletion and excess. The review covers from preclinical information about endogenous NO produced by nitric oxide synthase (NOS) to the potential therapeutic role of exogenous NO (inhaled nitric oxide, iNO). Moreover, the importance of NO in several clinical conditions in critically ill patients such as hypoxemia, pulmonary hypertension, hemolysis, cerebrovascular events and ischemia–reperfusion syndrome is evaluated in preclinical and clinical settings. Accordingly, the mechanism behind the beneficial iNO treatment in hypoxemia and pulmonary hypertension is investigated. Furthermore, investigating the pathophysiology of brain injury, cardiopulmonary bypass, and red blood cell and artificial hemoglobin transfusion provides a focus on the potential role of NO as a protective molecule in multiorgan dysfunction. Finally, the preclinical toxicology of iNO and the antimicrobial role of NO—including its recent investigation on its role against the Sars-CoV2 infection during the COVID-19 pandemic—are described.
Take home message
Nitric oxide (NO) is a key molecule involved in the vascular homeostasis and a key player in maintaining multiorgan viability. The therapeutic role of inhaled NO ranges from cardio-cerebrovascular to respiratory diseases to antimicrobial properties.
Similar content being viewed by others
Introduction
In the 1980s, nitric oxide (NO, nitrogen monoxide or nitrogen oxide) was considered just a toxic molecule, an environmental pollutant found in cigarette smoke and smog. It was known to have a role in destroying the ozone layer, and as a suspected carcinogen [1] and a precursor of acid rain. However, in the early 1990s an increasing amount of evidence showed that NO is an essential player in pathophysiology of mammals. Its activity was discovered to be fundamental in the brain, arteries, immune system, liver, pancreas, uterus, peripheral nerves, and lungs. In 1992, NO was declared the “Molecule of the Year” [2] and “initiated a new chapter in biomedical research” as Prof. Sten Lindhal stated in 1998, when the Nobel Prize in Physiology and Medicine was awarded to Robert Furchgott, Louis Ignarro and Ferid Murad for discovering NO’s role as a cardiovascular molecule [3].
NO in the environment
NO is a colorless and odorless gas, poorly soluble in water [4]. Atmospheric NO concentration ranges between 10 and 500 parts per billion (ppb). However, its concentration is estimated to rise up to 1.7 parts per million (ppm) in highly polluted areas [5]. Further, cigarettes, combustion and lightning can significantly increase NO concentration in the surrounding environment [6]. NO is an unstable gas and undergoes oxidation to more toxic nitrogen oxides (e.g., NO2, N2O4).
NO delivery systems
NO can be generated and delivered in different ways [7]: 1. pressurized cylinders are the most widely used system to store NO, delivery is regulated by sensors to control the concentrations of NO and NO2; 2. electric NO generators produce NO from ambient air using high-voltage electrical discharge to ionize air, which leads to the formation of NO and other byproducts filtered by a scavenging system; 3. chemical generators can produce NO by the reduction of NO2 by ascorbic acid; 4. NO-releasing solutions, release NO under specific chemical conditions; and 5. solid nanoparticles contains either NO or an inactive NO precursor in a stable form that releases NO in a controlled manner.
Furthermore, endovenous NO-donors (e.g., nitroglycerin, sodium nitroprusside) are commercially available drugs aimed at administering NO although not selectively (i.e., into the bloodstream)—like in the case of iNO. Despite mentioning NO-donors in this review to clarify certain mechanisms of action of NO, a comprehensive description of systemic NO-donors is out of the scope of the present review and may be consulted in other scientific reports [8, 9].
Nitric oxide synthase (NOS)
Endogenous NO is produced by nitric oxide synthases (NOS), the enzymes that catalyze nicotinamide adenine dinucleotide phosphate (NADPH) and tetrahydrobiopterin (BH4) dependent oxidation of L-arginine to L-citrulline. NO is one of the end-products of the reaction [10, 11]. The cofactor BH4 is essential for NOS to generate NO since its absence causes NOS to shift from a dimeric to a monomeric form, thus becoming uncoupled [12].
Three NOS isoforms were discovered in humans: neuronal (nNOS or NOS I), inducible (iNOS or NOS II) and endothelial (eNOS or NOS III).
eNOS is the constitutive form in endothelial cells, thus it is the main contributor to vascular NO levels in physiological conditions. eNOS is a dimer containing two identical monomers with a reductase domain for NADPH and an oxidase domain for L-arginine.
nNOS is a constitutively expressed form of NOS that was first found in neurons [13]. It is also present in other tissues including vascular smooth muscle cells, fibroblasts, endothelial cells and cardiomyocytes. nNOS activity is regulated by calcium/calmodulin interaction and it is susceptible to feedback inhibition by NO [14]. This feature guarantees pulsatile NO production instead of generating sustained low levels, a process linked to its role in synaptic transmission [15]. Moreover, recent evidence showed that nNOS-derived NO may play an important role in vascular physiology [16].
iNOS activity is mainly regulated by gene transcription and is modestly sensitive to NO-dependent autoinhibition, moreover its action is Ca2+-independent compared with the other forms of NOS [17]. iNOS is widely expressed in mammalian cells, particularly in immune cells (such as dendritic cells, NK cells, mast cells and phagocytic cells including monocytes, macrophages, microglia, Kupffer cells, eosinophils, and neutrophils) [18]. NO has a complex function in immune cells since it serves as an antimicrobial agent via NO-derived peroxynitrite (ONOO–), a reaction product of ·NO and O2–, as well as an immunomodulator via numerous pathways of lymphocyte inhibition and apoptosis [19]. Extensively studied in various pathophysiological processes [20], iNOS expression is described also in airway epithelium [21,22,23] under inflammatory stimuli and in blood vessels [24], where iNOS activation can lead to excess NO concentration and severe impairment of vascular function due to reduced NO sensitivity [25].
NO physiology
The role of NO as major mediator of vasodilatation has been well established since 1987 thanks to the work of Ignarro et al. [26] and Palmer et al. [27] The groups in two independent studies identified in NO the specific molecule previously known as endothelium-derived relaxing factor (EDRF). Moreover, a variety of nitro-vasodilators (e.g., nitroglycerin, sodium nitroprusside) is responsible for smooth muscle relaxation via cGMP synthesis, a process attributed to the release of NO [28, 29]. Endothelial cells in healthy blood vessels secrete NO tonically and enhance NO production dynamically in response to an increased shear stress, by locally controlling the organ perfusion according to changes of blood flow [30].
Although discovered as a vasodilator, NO exerts an important protective role on endothelium and guarantees vascular homeostasis [31]. Precisely, NO reduces vascular smooth muscle proliferation [32], platelet aggregation [33, 34] and leukocyte binding to endothelium [35, 36]. Furthermore, NO limits oxidative phosphorylation in mitochondria, a function that may be involved in the regulation of cell bioenergetics and apoptosis [37].
Most of the effects of NO in the cardiovascular system are mediated by the activation of the enzyme-soluble guanylate cyclase (sGC), which catalyzes the formation of the second messenger cyclic guanosine monophosphate (cGMP) from guanosine-5′-triphosphate (GTP): the activation of GMP‐dependent protein kinase G (PKG) leads to vascular relaxation (Fig. 1A).
NO biosynthesis and eNOS uncoupling. Endogenous NO is produced by NOS by the oxidation of l-arginine to l-citrulline + NO (NADPH and BH4-dependent reaction). NO is one of the end-products of the reaction. Most of the effects of NO in the cardiovascular system are mediated by the activation of sGC, which catalyzes the formation of the second messenger cGMP from GTP. The activation of GMP‐dependent PKG leads to vascular relaxation (A). Several circumstances may alter eNOS activity causing the reduction of NO levels and triggering the production of superoxide instead of NO, a process defined as “eNOS uncoupling”. For example, the depletion of eNOS cofactor BH4, l-arginine deficiency, and increase in endogenous eNOS inhibitor ADMA lead to eNOS uncoupling. This process is largely deleterious and has been linked to endothelial dysfunction, ROS increase and other vascular pathologies. Moreover, NO bioavailability is reduced by free oxy-Hb. B NO: nitric oxide; NOS: nitric oxide synthase; sGC: soluble guanylate cyclase; cGMP: cyclic guanosine monophosphate; GTP: guanosine-5′-triphosphate; PKG: Protein Kinase G; BH4: tetrahydrobiopterin; ADMA: asymmetric dimethylarginine; oxy-Hb: oxyhemoglobin
Soluble guanylate cyclase (sGC) is a heme-containing protein composed of an α and β subunits. The presence of heme results in a 100-fold increase of the enzyme activity after stimulation with NO, whereas basal enzyme activity is low without heme and does not change regardless the addition of NO [38].
NO may directly modulate other signaling systems, including nitrosylation of a wide range of proteins, thus modifying their biological activity [39]. The target proteins include the transcription factor nuclear factor kinase-B (NFκB), cell cycle-controlling proteins, and proteins involved in the generation of tissue factor [40]. Moreover, since NO is rapidly sequestered from the circulation, bound and inactivated via redox activity to nitrate (NO3−) by heme-iron of hemoglobin (Hb) [41], an additional mechanism to preserve NO bioavailability is necessary: NO is activated in vivo, requiring oxidation of NO to NO+, to allow its reaction with thiols [42, 43]. S-Nitrosothiols (SNO) and in particular S-nitroso-hemoglobin (SNO-Hb) are resistant to heme, thus maintaining its ability to perform vasodilatory activity [44]. Accordingly, the systemic hypoxic vasodilation observed by Guyton in the 1960s [45] is better explained by SNO-Hb itself: NO is released from SNO-Hb during deoxygenation in the microcirculation to regulate vessels directly, thus diverting blood flow to the tissues with increased oxygen demand. Furthermore, while SNO serves as the main source of NO in the microcirculation, SNO itself has a proper bioactivity that is carried out regardless sGC/PKG [46,47,48]. S-nitrosylation is now recognized as a fundamental post-translational modification and as a major key player in the NO bioactivity [49].
NO toxicology
The toxicology of NO is complex since numerous NO-donors show significant side effects, particularly hypotension given their ability to vasodilate [50]. Moreover, as stated in the section on the ischemia–reperfusion syndrome (IRS), some studies showed deleterious effects of NO on brain damage [51,52,53].
Inhaled nitric oxide (iNO) has a complex interaction between the pharmacological properties and toxic effect [54]. Some of the toxic effects are mediated by its second messenger cGMP that, among other roles, can modulate DNA synthesis and decreases cellular proliferation. The antiproliferative effects of NO has been demonstrated in several systems, including vascular smooth muscle and human airway smooth muscle cells in vitro [55, 56]. Further studies are necessary to understand if this effect is beneficial or deleterious in hypoxic pulmonary vasoconstriction (HPV). The potential genotoxic effects of NO is also a concern since chromosomal aberrations in lung cells in rats are reported [57]. Similar results were obtained in human lymphoblastoid cells in vitro following nitric oxide treatment [58].
Nitric oxide also reacts with superoxide anion to form peroxynitrite (ONOO−), a highly reactive oxidant species [59]. Peroxynitrite can induce lipid peroxidation and inhibit mitochondrial respiration [60, 61]. Furthermore, it can also initiate DNA base modifications [62, 63]. Moreover, iNO can rapidly react with oxygen in the lung to form NO2, which is a potent pulmonary irritant that may alter the surfactant [64]. Trials on lambs and rats exposed to high doses of iNO (80 or 100 ppm, respectively) demonstrate surfactant dysfunction [65, 66].
iNO is able to exert its toxic effects outside the lung, despite the rapid inactivation by circulating Hb. In particular, iNO may cause vasodilation in extrapulmonary circulation [67], a process that may be related to the formation of S-nitroso-proteins that maintain NO biologically active [68]. Moreover, NO inhibits platelet aggregation and adherence to endothelial cells [69]. In rats, iNO (15 ppm) increased bleeding time and reduced platelet aggregation [70].
Finally, iNO can combine with hemoglobin to form met-Hb. Toxic levels of met-Hb are reached only when high dose of iNO are administered [71, 72], and a rapid clearance was demonstrated in rats and rabbits treated with iNO after the return to breathing air [73, 74].
NO pathophysiology
iNO may have diverse clinical applications thanks to its ubiquitous role in organ function and viability. Despite extensive pre-clinical and clinical literature is available, a lot of work should be done yet to investigate iNO potential before targeting clinical trials. A summary of the highest level of evidence so far available about the iNO potential for clinical applications and highlights on research gaps before trialing in the absence of clinical trials in each specific area of research are reported in Table 1.
Endothelial function and vascular homeostasis
Endothelial dysfunction is a disorder characterized by an imbalance between vasodilating, antimitogenic and antithrombogenic molecules and others with vasoconstricting, prothrombotic, and proliferative properties [75, 76]. As already illustrated in the previous section, NO is one of the key substances involved in vasodilation, platelet aggregation, leukocyte adhesion activation and smooth muscle cell proliferation.
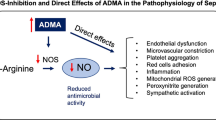
Several circumstances can alter eNOS activity causing the reduction of NO levels and triggering the production of superoxide instead of NO, a process defined as “eNOS uncoupling”. For example, the depletion of eNOS cofactor tetrahydrobiopterin (BH4), l-arginine deficiency and increase in endogenous eNOS inhibitor asymmetric dimethylarginine (ADMA), lead to eNOS uncoupling [77]. This process is largely deleterious and has been linked to endothelial dysfunction, ROS increase and other vascular pathologies [78]. Particularly, plasma ADMA levels are increased in humans with hypercholesterolemia, atherosclerosis, hypertension, chronic renal failure, chronic heart failure and insulin resistance [79]. Moreover, NO bioavailability is reduced by free oxy-Hb (see “18” section) (Fig. 1B).
Studies on eNOS knock-out mice showed that eNOS mediates basal vasodilation [80], promotes angiogenesis and helps wound healing [81]. Moreover, the vascular protective role of NO is confirmed by studies on eNOS polymorphisms associated with reduced NO production: an association of low NO synthesis was found with coronary spasm [82], hypertension [83], pre-eclampsia [84], diabetic nephropathy [85] and retinopathy [86], and vascular erectile dysfunction [87]. Thus, endothelial dysfunction contributes to the pathogenesis of cardiovascular disease and there is strong clinical evidence that loss of NO bioavailability is a crucial manifestation of endothelial dysfunction [88].
Several methods to measure endothelial dysfunction have been proposed based on the concept that healthy arteries dilate consequently to reactive hyperemia (flow-mediated vasodilatation) or after pharmacological stimuli. In disease states, this mechanism is reduced or absent. Since endothelial dysfunction is characterized by the inability to produce endogenous NO, to discriminate endothelium-independent from endothelium-dependent responses, exogenous NO-donors (e.g., sodium nitroprusside) or non-NO-donors vasodilators (e.g., acetylcholine (Ach), which is the molecule responsible of endogenous vascular NO production and consequent vasodilatation) can be applied. Consequently, a vasodilatory response after NO-donors and absence of response after acetylcholine is typical of endothelial dysfunction. Impaired endothelial-independent function is indeed associated with structural vascular alterations rather than changes in the endothelial function [89].
In 1993, Wessel et al. investigated whether cardiopulmonary bypass may induce pulmonary endothelial dysfunction and then lead to pulmonary hypertension in children with congenital heart disease undergoing surgical repair. To test their hypothesis, the authors explored the effects of iNO, as an endothelial-independent smooth muscle relaxant, and Ach, as an endothelial-dependent vasodilator. The authors demonstrated that Ach failed to reverse pulmonary hypertension. In contrast, iNO reversed increased pulmonary pressures bypassing the impaired endothelial signaling pathway of Ach. This confirmed the hypothesis that endothelial dysfunction seems to be the cause of the altered endogenous NO release. Furthermore, plasma levels of cGMP were unchanged after Ach infusion but increased more than threefold during pulmonary vasodilation with iNO. This finding was consistent with the hypothesized role of cGMP as the second messenger of effective smooth muscle relaxation in this process [90].
Ischemia–reperfusion syndrome
Hypoxia-induced release of NO is one of the major determinants of microvascular blood flow modifiers [91,92,93]. NO may exert this function by Hb S-nitrosylation at Cys93 of the β-chain [49]. The release of SNO from the deoxygenated structure of Hb is supported by data showing that wild-type mice exhibit elevated muscle blood flow after brief ischemia (reactive hyperemia), a mechanism markedly impaired in mice expressing Hb with a single point mutation in Cys93 of the β-chain, and so unable to carry SNO [94]. Also, recombinant Hb unable to carry NO was associated with increased cardiac injury and mortality in an animal model of myocardial infarction [95].
Since NO has homeostatic and protective roles on endothelium, several studies tried to assess the putative beneficial effect of NO on different organs that may be potentially prone to develop injury consequent to ischemia. Both NO-donors and iNOs were studied in IRS. However, while the mechanism of NO delivery of the former is easy to be understood, iNO is rapidly inactivated by Hb-mediated oxidation in the circulation (see also the section “18”) and may not reach the target organ. However, long-lived tissue metabolites may account for the preconditioning effects of iNO itself [96].
In animal models of myocardial IRS basal NO release was significantly decreased after myocardial ischemia and reperfusion compared to non-ischemic control [97]. Further detailed information about myocardial IRS and on the role of NO in myocardial protection are reported in the section “16” below.
Similar results were obtained on lung IRS in rats where iNO decreased inflammation and vascular permeability (i.e., as seen by a decrease in extravascular albumin accumulation) and prevented the increase in lung wet-to-dry weight ratio [98]. Furthermore, in a rodent model of ex vivo lung perfusion, iNO administration before and after lung retrieval improved lung function by reducing wet-to-dry weight ratio and pulmonary vascular resistance, guaranteeing better oxygenation, increasing lung tissue levels of cGMP, and by decreasing lung tissue tumor necrosis factor alpha (TNF-α) and iNOS [99].
Also, intestinal IRS in animal model may benefit from 80 ppm iNO since it abrogates IRS-induced perfusion reduction, the increase in leukocyte rolling, adhesion, and emigration, and the endothelial dysfunction [100]. Moreover, exogenous NO (both inhaled or via NO-donors) promotes hepatic tissue blood flow after reperfusion, decreases neutrophil accumulation and prevents the excessive production of iNOS in hepatic IRS in animals [101].
Despite significant effects in mammals, little evidence of beneficial effects of iNO in human models of IRS has been demonstrated. iNO seems to reduce pro-inflammatory cytokines after tourniquet application during knee surgery[102] and 80 ppm iNO significantly decreases hospital length of stay and accelerates the normalization of serum transaminases and coagulation times after orthotopic liver transplantation [103]. We further suggest that the time of iNO administration might play a key role in relation to the different pathogenetic stages of IRS. This may be crucial to interpret the findings on outcome in clinical studies.
NO and the brain
Cerebral ischemia and stroke
Cerebral blood flow (CBF) is tightly regulated since neuronal activation requires large amounts of energy. Autoregulation and neurovascular coupling are the two main determinants of CBF, and both are affected by NO [104]. Autoregulation maintains CBF stable regardless of the changing of cerebral perfusion pressure. Inhibition of NO synthesis in eNOS knock-out mice results in the right shift of the hypotensive portion of the cerebral autoregulatory curve, thus impairing CBF at lower perfusion pressures [105]. Neurovascular coupling is the process by which the neurovascular unit (i.e., a functional structure composed by neurons, glial cells and blood vessels) modulates local CBF according to local metabolic demands [106]. nNOS inhibition in rats causes significant attenuation of the cerebral blood flow response to the somatosensory stimulation, suggesting disruption of neurovascular coupling [107].
Despite its fundamental role in brain physiology, NO activity in cerebral ischemia is extremely complex due to the interaction between the toxic effects of nitrates, the release of free radicals, and the neuroprotective effects on the vascular bed homeostasis [51,52,53]. iNOS can be stimulated by stress, inflammation, and infection. Under these conditions, NO can be generated in large quantities and has detrimental effects on the CNS increasing permeability of the blood–brain barrier [108].
The role of NO in stroke is controversial since a multitude of animal studies reported both neurotoxic and neuroprotective effects. Most of the neuroprotective effects of NO as reduction of infarct size in models of middle cerebral artery occlusion are associated with eNO [53, 105]. In contrast, the neurotoxicity is primarily related to nNOS and iNOS, by a mechanism related to the production of nitrates and the release of free radicals [109, 110]. However, in recent years evidences showed that overall NO has a predominant beneficial role in stroke [51], and iNO showed to be effective in reducing the cerebral infarct size in rodents models [111,112,113].
Subarachnoid hemorrhage
An interesting implication of NO in the pathogenesis of delayed cerebral ischemia following subarachnoid hemorrhage (SAH) has been proposed, suggesting both eNOS and nNOS dysfunctions are among the mechanisms of the disease [114]. Driven by immunological and nonimmunological processes, red blood cells (RBCs) of the subarachnoid clot hemolyze resulting in delayed occurrence of cell-free Hb in the cerebrospinal fluid. Elevated concentrations of cell-free Hb in the cerebrospinal fluid are associated with a delayed ischemic neurological damage in patients with subarachnoid hemorrhage [115]. In addition, delayed cerebral ischemia is associated with reduction of NO levels in the cerebrospinal fluid [116]. The NO-scavenging effect of cell-free Hb might disrupt the endothelial NO signaling of cerebral arteries leading to vasoconstriction and consecutive delayed vasospasm [117, 118]. Systemic NO-donors have shown a role in delayed cerebral ischemia prevention in animal models [119, 120]. Furthermore, sequestration of cell-free Hb in large hemoglobin–haptoglobin complexes prevented the interaction of cell-free Hb with endothelial and tissue NO and restored physiological NO signaling in cerebral vasculature in an experimental setting [121].
Interestingly, recent findings showed that early cerebral ischemia after SAH may be due to constriction of pial arterioles [122,123,124]. In a rodent model of induced SAH, iNO significantly reduced early micro-vasospasms, while only having limited effect on large artery spasms. This resulted in less brain-edema formation, less hippocampal neuronal loss, mortality reduction, and improvement of neurological outcome [125].
Traumatic brain injury
Inappropriate inflammatory response is a major determinant in secondary brain damage after traumatic brain injury (TBI) [126]. The mechanism behind the hazardous increase of NO production in TBI is the upregulation of iNOS [127]. The exaggerated NO levels in the brain contribute to the TBI-associated glutamate cytotoxicity, including the pathogenesis of neuronal apoptosis and mitochondrial dysfunction [128].
However, opposite results were obtained in animal models of TBI. TBI may increase arginase activity, which competes with eNOS for L-arginine, thus limiting NO production [129]. Moreover, iNOS-deficient mice showed enhanced oxidative stress compared to the control group [130], suggesting the antithetical effect of this molecule in the brain.
Nevertheless, iNO exhibited a significant role in preserving cerebral autoregulation and secondary brain injury after TBI in murine [131] and porcine models [132, 133].
NO and the cardiovascular system
Cardiac arrest
Cardiac arrest (CA) is the prototype of a global IRS of the whole body. Organs with a high metabolic demand—such as brain and heart—are particularly prone to IRS. As already illustrated in the former section of the review, NO seems to have a protective role in preclinical models of IRS. Similarly, several pharmacological interventions that increase NO bioavailability have been reported to improve outcomes in preclinical CA models [134]. Breathing 40 ppm NO for 23 h after potassium-induced CA in mice prevented neurological and cardiac dysfunction. Indeed, iNO attenuated brain edema as measured by magnetic resonance imaging at 24 h after resuscitation, while decreased apoptosis of hippocampal neurons and induction of inflammatory cytokines in the cortex. Moreover, treatment of mice with iNO markedly improved the survival rate from 31 to 85% compared to air breathing controls [135]. Finally, among the mechanisms responsible for the beneficial effect of NO in CA, sGC seems to be critically important since deletion of its 1α subunit abolished the protective effects of iNO on neurological function and survival after CA [135].
In addition, pharmacological prevention of the reduction of S-nitrosylated proteins in brain occurring after CA improves the survival rate in mice with ischemic brain injury [136]. In humans the literature is limited; however, in a pilot study on patients with intra-hospital CA, iNO was associated with significantly higher rates of survival, but no difference in favorable neurologic outcome was observed [137]. As a note of interest, preclinical evidence suggests that how cardiopulmonary resuscitation is delivered (i.e., mechanical versus manual chest compression) may decrease oxygenation after the return of spontaneous circulation because of lung edema [138,139,140]. The role of iNO in this setting to potentially improve oxygenation might be a field of future investigation.
Myocardial infarction
As already illustrated in the IRS section, NO deficiency is associated with tissue damage after reperfusion. This phenomenon is particularly relevant in a myocardial ischemia model [97].
Interestingly, the pharmacological correction of NO depletion has demonstrated better myocardial protection, which was defined as reduced ischemic area and neutrophil adherence, in a trial of myocardial IRS in dogs (i.e., NO-donor vs. placebo) [141]. Furthermore, myocardial IRS is exacerbated in the absence of eNOS [142] and—in contrast—the cardiomyocyte-specific eNOS overexpression protects myocardium [143].
Furthermore, iNO administered during myocardial IRS at 40–80 ppm reduces the infarct size and improves the left ventricular function in mice [144]. Similar results were obtained in a porcine model of myocardial infarction treated with iNO at 80 ppm 10’ before reperfusion during the subsequent 4 h. iNO improved the microvascular perfusion, reduced the infarct size, and reduced the myocardial leukocyte infiltration [145].
In humans, the inhalation of NO at 80 ppm for 4 h after reperfusion in STEMI did not reduce the infarct size at 48–72 h [146].
NO and hemolysis
Hemolysis
Heme-containing proteins avidly bind to NO. Under physiological conditions, NO scavenging is slow because Hb is confined to inside red blood cells (RBCs). However, during intravascular hemolysis, free-Hb in plasma is able to rapidly bind to vascular NO, thus affecting vasomotor tone and consequently organ perfusion [147]. Particularly, this occurs by the di-oxygenation reaction of plasma oxy-Hb (Fe2+) with NO to form bio-inactive nitrate and met-Hb (Fe3+) [148].
In 2005, Minneci et al. demonstrated that intravascular hemolysis in a canine model produces dose-dependent systemic and pulmonary vasoconstriction [149]. In order to understand the mechanism behind the reduced vasoreactivity in the presence of free-Hb, the authors showed that the delivery of 80 ppm of iNO reverted the vasoconstrictive effect of plasma Hb. These observations indicate that the acute pulmonary and systemic vasoconstriction by intravascular hemolysis occurs secondarily to the accelerated di-oxygenation reaction of plasma oxy-Hb with NO to form bio-inactive nitrate and met-Hb. However, the concentration of plasma Hb itself is not the single parameter responsible to this effect because only the oxidizing biochemical form (oxy-Hb) is able to bind to NO, subsequently causing vasoconstriction and becoming vascular inactive as met-Hb.
In their same manuscript, Minneci et al. also demonstrated that the amount of hemolysis is associated with impairment of renal function assessed by a reduced creatinine clearance at 6 h from the insult. This evidence was suggested to unveil a potential link between the onset of hemolysis and organ perfusion: the greater the oxy-Hb concentration, the greater the vasoconstriction, the greater the reduction in organ perfusion with the consequent drop in creatinine clearance. Notably, creatinine clearance was restored in the hemolysis group treated with iNO, confirming its role in oxy-Hb inactivation (Fig. 2).
NO scavenging in hemolysis. The di-oxygenation reaction: during intravascular hemolysis in human disease, oxy-Hb (Fe2+) is able to rapidly bind NO, to form bio-inactive NO3− and met-Hb (Fe3+). The NO scavenging causes consequently vasoconstriction. Exogenous NO can prevent this phenomenon by minimizing the scavenging of endogenous NO. The graph represents the different light absorption wavelengths of oxy-Hb and met-Hb. NO: nitric oxide; Hb: hemoglobin; RBC: red blood cell; NO3−: nitrate
Intravascular sequestration of cell-free Hb by the Hb-binding protein haptoglobin was shown to protect vascular NO signaling [150]. Interestingly, in the presence of endothelial dysfunction such as in models of diabetes mellitus or hyperlipidemia in mice, haptoglobin was not able to prevent vasoconstriction [151]. Because endothelial dysfunction enhances vasoconstriction due to NO scavenging by cell-free Hb, this may suggest that in contrast to supplementation of NO, sequestration of cell-free Hb by Hb-binding molecules is not effective to prevent vasoconstriction in the presence of endothelial dysfunction [151,152,153].
In 2004, Gladwin et al. found an association between sickle cell disease and pulmonary hypertension, a process that may be due to NO scavenging by plasma oxy-Hb [154]; these findings were confirmed by more recent studies [155]. The protective effect of exogenous NO in sickle cell disease has been hypothesized and it may be consequent to NO restoration, red cell adhesion reduction and vaso-occlusion prevention [156]. However, the role of hemolysis in the pathogenesis of pulmonary hypertension in patients with sickle cell disease remains controversial [157].
Furthermore, pathophysiological hemodynamic changes during acute pulmonary thromboembolism may be partly caused by increased Hb decompartmentalization and consequent augmented nitric oxide consumption resulting in vasoconstriction [158].
Cardiopulmonary bypass-associated hemolysis
Cardio-pulmonary bypass (CPB) is known to be associated with increased pulmonary vascular resistance (PVR) [90, 159] and systemic vascular resistance (SVR) [160] particularly in the first hour after the procedure [161]. Hemolysis-induced perturbations in microcirculatory blood flow and subsequent hypoperfusion or even ischemic damage should be recognized as an important risk factor for organ injury development in patients undergoing cardiovascular surgery [162, 163].
In 2016, Rezoagli et al.[164] found that a prolonged duration of CPB (≥ 140 min) was associated with higher levels of hemolysis and both systemic and pulmonary vasoconstriction at 15 min after CPB. The investigators reported an independent linear correlation between the change of nitric oxide consumption and the change of systemic and pulmonary vascular resistance within 4 h after CPB. The length of the procedure was directly related the level of plasma Hb, and consequently to NO consumption [162], resulting in higher pulmonary and systemic vascular resistances. Reduction of NO bioavailability during CPB is not only consequent to increase in free-Hb NO consumption; also, endothelial dysfunction plays a role during this procedure impairing endogenous NO production [90].
Interestingly, the enhanced NO consumption and reduced synthesis after CPB also play a role in organ damage such as acute kidney injury (AKI) and intestinal injury [165], a process linked to the reduction of organ blood flow due to vasoconstriction already studied by Minneci as previously described. Moreover, early exogenous NO administration during CPB, improving the oxidation of oxy-Hb to met-Hb and therefore reducing systemic vasoconstriction, reduces the risk of AKI [166]. A clinical trial is currently ongoing to evaluate whether administration of 80 ppm iNO during CPB and for 24 h after surgery reduces the risk of AKI in patients with endothelial dysfunction [167].
Blood transfusion-associated hemolysis
Transfusion of erythrocytes stored for prolonged intervals is associated with increased morbidity (e.g., increased risk of AKI, sepsis and duration of mechanical ventilation) and mortality [168, 169]. Because storage affects the integrity of the red cell membrane, numerous erythrocytes hemolyse during storage or shortly after transfusion which is known as the so-called “storage lesion” [170, 171]. Therefore, transfusion of prolonged stored red blood cell leads to an increase of plasma Hb with consecutive scavenging of endogenous NO resulting in systemic and pulmonary vasoconstriction [151, 171, 172].
In their work, Berra et al. [172] introduced the possibility of reducing the adverse effects after transfusion of 40-day-stored packed RBCs by supplementing iNO: in obese volunteers, breathing 80 ppm NO prevented the increase of pulmonary artery pressure after transfusion of prolonged stored blood. Similarly, inhalation of 80 ppm NO prevented the vasoconstrictor response of older RBC infusions in lambs [173].
Moreover, pre-treatment of RBCs with NO-donors seems to guarantee better RBC storage quality, reducing the amount of hemolysis (measured as LDH activity) and the depletion of vital metabolites (such as 2,3-diphosphoglycerate) [174]. Similar results were obtained with RBC pre-transfusion treatment with gaseous NO or NO-donors [175].
Cell salvage devices are widely used during surgery when a consistent blood loss is expected. Hemolysis in these circumstances is a major concern due to the mechanical trauma of washing autologous blood [176, 177]. Although modern cell salvage systems can remove the majority of free-Hb during washing, they do not select between intact RBCs and damaged RBCs, which are prone to delayed hemolysis in vivo [178]. Exogenous NO may play a key role to prevent endothelial dysfunction with impaired vasorelaxation because of delayed hemolysis in vivo after administration of autologous blood by cell salvage [162].
Artificial hemoglobin
Blood transfusion is a common procedure performed during clinical practice and, despite all of the measures taken to ensure its safety, there are known risks associated with transfusions [178]. In addition, the use of blood products is limited by further technical issues such as product availability, need for compatibility testing, and storage and transport requirements. Moreover, there are individuals who do not accept blood transfusions. Therefore, great efforts were made to develop alternative agents that may reliably and safely replace blood. One of the most studied type of artificial blood substitute is the hemoglobin-based oxygen carrier (HBOC). HBOCs use free synthetic Hb to carry oxygen throughout the body. Due to its high toxicity, the FDA has not approved any HBOC for clinical use in the United States [179]. Since an HBOC is in fact a free-Hb complex, vasoconstriction induced by artificial blood transfusion seems to be determined by a similar scavenging mechanism of endogenous NO as in plasma-free Hb models. In 2008, Yu et al. showed in animal models that the administration of iNO could reverse systemic and pulmonary HBOC-induced vasoconstriction [152]. In 2010, the investigators further reported a relation between endothelial dysfunction and the severity of HBOC-induced side effects. Overall, these results support the hypothesis of an inverse relation between vascular NO levels and the severity of endothelial dysfunction [153].
NO and the lungs
Pulmonary shunt
HPV was first identified in 1894 by Bradford [180] and later further characterized by Von Euler in 1946 [181]. HPV is the consequence of the constriction of small intrapulmonary arteries in response to alveolar hypoxia [182]: this is the cornerstone physiological mechanism owing to lung perfusion–ventilation matching. Vasoconstriction in response to hypoxia is the hallmark of the pulmonary vasculature. In contrast, systemic vessels dilate in response to hypoxia in order to increase tissue oxygen delivery [183, 184].
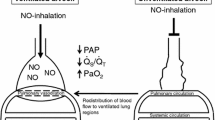
Extensive literature about the effect of NO during hypoxemia and HPV was provided by Professor Warren M. Zapol. The start of his scientific contributions dates back almost 50 years ago during his physiologic studies on oxygen metabolism in Weddell seals, animals that can hold their breath for over an hour on dives up to 600 m deep, tolerating a high grade of hypoxemia, high pressure and severe cold conditions [185]. The founding hypothesis was the need to treat respiratory failure to reverse hypoxia and enhance survival. However, systemic vasodilators had the opposite effect on arterial oxygenation by non-selectively dilating the pulmonary and systemic vascular bed [186]. A great advancement for the scientific community in the field of NO was the understanding of the physiological mechanism underlying its inhalation thanks to Dr. Zapol intuition. In a sheep model of thromboxane-induced and hypoxia-induced pulmonary hypertension in 1991, Dr. Frostell and Dr. Zapol with colleagues demonstrated that iNO (5–40 ppm) reversed pulmonary hypertension within 3 min; systemic vasodilation did not occur and pulmonary hypertension resumed within 3–6 min of ceasing NO inhalation [187]. Similar effects were obtained in humans with chronic pulmonary hypertension, confirming the selectivity of iNO for the pulmonary vasculature, without affecting mean systemic arterial pressure [188, 189]. iNO acts selectively on the vasculature associated with ventilated lung units: just those specific vessels that are exposed to the inhaled gas diffusing across the alveolar-capillary membrane. Selective dilatation of these vessels improves ventilation–perfusion matching. This effect has gained importance in the treatment of severe hypoxemia in patients with acute respiratory distress syndrome (ARDS) [190,191,192,193], the prototype condition of perfusion of dis-ventilated alveoli [194, 195]. Moreover, the rapid clearance of NO by Hb guarantees the absence of systemic hypotension from systemic vasorelaxation.
In 1992, Roberts et al. [196] and Kinsella et al. [197] demonstrated that iNO improved oxygenation in persistent pulmonary hypertension of the newborn (PPHN) where the lack of surfactant determines hypoxemia because of alveolar collapse and reduced ventilated alveolar units. Then, the reduction of mortality in patients with PPHN treated with iNO confirmed the robust pathophysiological link between iNO treatment and PPHN [198].
The In 1993, Rossaint et al. demonstrated that in patients with ARDS, a disease associated with lung heterogeneity and a high degree of right to left shunt [199], iNO reduces pulmonary vascular pressure and right ventricle overload and, by specifically dilating oxygenated vessels, iNO diverts pulmonary blood flow toward ventilated alveoli, therefore reducing pulmonary shunt and increasing arterial oxygenation [200] (Fig. 3). While hypoxemia-induced vasoconstriction reduces blood flow to non-ventilated areas therefore increasing pulmonary vascular resistance, iNO dilates vessels of better ventilated areas thus reducing pulmonary vascular resistance entering from the alveoli to the pulmonary vessels. These mechanisms are additive and allows for the reduction of pulmonary shunt and the increase of arterial oxygenation diverging pulmonary blood flow to more ventilated lung units. The selective effect of iNO for ventilated areas may be mimicked by using intratracheal NO-donors since their administration showed potential benefit on hypoxemia in ARDS in animal models, despite, no impact on mortality was reported [201, 202].
iNO reversal of hypoxemia and pulmonary hypertension during HPV. iNO is a pulmonary selective vasodilator. It diffuses selectively from ventilated alveoli to the adjacent pulmonary capillaries. This reduces PVR and the right ventricle afterload. The selective vasodilation of oxygenated vessels diverges pulmonary blood flow towards the ventilated alveoli. As a consequence, pulmonary shunt is reduced and arterial oxygenation is increased. In physiologic conditions, most of the alveoli are well ventilated and perfused, as low PVR ensures that a wide pulmonary capillary bed is recruited (A). If some of the alveoli are poorly or not ventilated (e.g., atelectasis, pneumonia), the pulmonary capillaries that perfuse those alveoli constrict because of HPV. The increased PVR leads to a consequent reduction of the available pulmonary vascular bed. This limits the blood perfusion of the poorly/not ventilated lung areas then limiting V/Q mismatch and pulmonary shunt (B). The administration of iNO in the presence of HPV increased the vasodilation of pulmonary vessels that are normally ventilated. This condition reduces PVR and reverses hypoxemia by diverging the blood flow to ventilated areas, thus reducing V/Q mismatch and pulmonary shunt (C). The PAO2-PACO2 graph below, represents the partial pressure of the alveolar gases in each of the conditions previously described. In physiologic conditions, the V/Q is optimal (A arrow); when some the alveoli are not ventilated, hypoxemia emerges because of pulmonary shunt despite the compensatory mechanism of HPV (B arrow). This condition may be partially reverted by the administration of iNO (C arrow). The bottom panel was adapted from West JB, Luks AM. West’s Respiratory Physiology. The Essentials. Tenth Edition. Wolters Kluwer, 2015. PAO2: alveolar pressure of O2; PACO2: alveolar pressure of CO2; V/Q: ventilation–perfusion ratio; PVR: pulmonary vascular resistance; HPV: hypoxic pulmonary vasoconstriction
However, iNO did not show clear mortality benefits in ARDS [203]. Further, some randomized studies failed to demonstrate sustained benefits on oxygenation [191] and iNO may worsen oxygenation at high doses [204].
The use of iNO is limited in the clinical practice [205]. Current guidelines do not recommend iNO in ARDS [206] or provide a weak recommendation against its use [207], since no large phase III randomized controlled trials are available on the use of iNO in ARDS [208]. Available clinical studies in ARDS do not differentiate whether iNO may play a different role on: different etiologies [209], management [210, 211], coexisting comorbidities [212] and organ dysfunctions [213, 214] in ARDS; sex [215]; limitation of care [216]; and whether these may variably interplay on the effects of iNO on outcomes. Furthermore, not just the dose may matter [217]. The timing of iNO administration and the duration of the iNO treatment are yet to be explored [218]. This makes the clinical evidence available not conclusive so far. Hopefully, the current insights of the ARDS stratification into phenotypes—that are biologically and clinically different features within the same definition of ARDS [219, 220]—may help the understanding of the effects of numerous pharmacological treatments in ARDS including iNO [221].
NO and sepsis
NO production is dysregulated in sepsis: exaggerated NO production may be responsible for cardiac, macrovascular, and cellular dysfunction, while reduced eNOS activity is a key factor of microvascular dysfunction. The role of NO in sepsis is not part of the current review and was extensively recently presented by Lambden [222].
NO and COVID-19
SARS-CoV-2 antiviral effect of NO
NO is an antimicrobial agent. Its role was demonstrated on different viruses [223, 224] and other pathogens like bacteria, fungi, and protozoa [225,226,227,228]. NO antimicrobial activity was measured as a reduction in the cytopathic effect in vitro against SARS-CoV-1 in a concentration‐dependent manner as compared to placebo [229].
During the recent COVID-19 pandemic, the scientific productivity on SARS-CoV2 increased tremendously to better understand, treat and explore treatments that may defeat this disease [230]. Among different proposed therapeutic agents, the potential viricidal activity of NO on SARS-CoV-2 is under investigation. In vitro studies showed that the NO-donor S-nitroso-N-acetylpenicillamine inhibits SARS-CoV-2 replication. This effect correlates with both the delay and the prevention of the viral cytopathic effects in culture-type Vero E6 cells treated with NO. Akaberi and coworkers proposed that the inactivation of SARS-CoV-2 protease by S-nitrosylation is the key mechanism behind the therapeutic role of NO [231]. Other protective proposed mechanisms are the production of reactive nitrogen intermediates that inhibit the viral replication and restore the depleted endogenous NO, thus mitigating the prothrombotic and vascular complications of COVID-19 [232].
Conclusions
NO is a molecule with a key role in human life. Its role as a beneficial agent in governing balance in organ perfusion and viability seems to overcome its limited side effects. iNOS was first demonstrated to be effective in HPV and in PPHN. However, NOS dysfunction has been linked to numerous pathologic conditions associated with the impairment of vascular homeostasis, such as ischemia, hypoxia, hemolysis, and inflammation. Furthermore, several experimental studies have shown potential beneficial effects of supplementing NO in these pathologic conditions—both as systemic NO-donors and iNO. The potential application of iNO in different organ dysfunctions is under investigation in humans. These findings may significantly improve our knowledge and understanding of the molecular pathophysiology in specific diseases, as well as in complex syndromes such as IRS and sepsis, where a specific molecular target has not been identified. The promising findings about the use of NO in preclinical research supports the translation of these results in studies aimed at exploring the effect of NO on clinical outcomes, guiding technological advances such as the optimization of organ transplantations and the use of CPB, and allowing for the safer transfusion of RBCs and HBOCs by limiting their side effects.
Availability of data and materials
Not applicable.
Abbreviations
- ADMA:
-
Asymmetric dimethylarginine
- AKI:
-
Acute kidney injury
- ARDS:
-
Acute respiratory distress syndrome
- CA:
-
Cardiac arrest
- CBF:
-
Cerebral blood flow
- cGMP:
-
Cyclic guanosine monophosphate
- CPB:
-
Cardiopulmonary bypass
- EDRF:
-
Endothelium-derived relaxing factor
- eNOS:
-
Endothelial nitric oxide synthase
- GTP:
-
Guanosine-5′-triphosphate
- Hb:
-
Hemoglobin
- HBOC:
-
Hemoglobin-based oxygen carrier
- HPV:
-
Hypoxic pulmonary vasoconstriction
- iNO:
-
Inhaled nitric oxide
- iNOS:
-
Inducible nitric oxide synthase
- IRS:
-
Ischemia–reperfusion syndrome
- NFkB:
-
Nuclear factor kinase-B
- nNOS:
-
Neuronal nitric oxide synthase
- NO:
-
Nitric oxide
- NOS:
-
Nitric oxide synthase
- ONOO− :
-
Peroxynitrite
- PAH:
-
Pulmonary artery hypertension
- PaO2/FiO2 :
-
Partial oxygen pressure-to-fraction of inspired oxygen ratio
- PAP:
-
Pulmonary arterial pressure
- PKG:
-
Protein kinase G
- ppb:
-
Parts per billion
- ppm:
-
Parts per million
- PPHN:
-
Persistent pulmonary hypertension of the newborn
- PVR:
-
Pulmonary vascular resistance
- RBCs:
-
Red blood cells
- SAH:
-
Subarachnoid hemorrhage
- sGC:
-
Soluble guanylate cyclase
- SNO:
-
S-Nitrosothiols
- SNO-Hb:
-
S-Nitroso-hemoglobin
- SVR:
-
Systemic vascular resistance
- TBI:
-
Traumatic brain injury
References
Lala P, Chakraborty C (2001) Role of nitric oxide in carcinogenesis and tumour progression. Lancet Oncol. https://doi.org/10.1016/S1470-2045(00)00256-4
Culotta E, Koshland DE (1992) NO news is good news. Science. https://doi.org/10.1126/science.1361684
(1998) Award ceremony speech. NobelPrize.org. In: https://www.nobelprize.org/prizes/medicine/1998/ceremony-speech/
Fukuto JM (1995) Chemistry of nitric oxide: biologically relevant aspects. Adv Pharmacol. https://doi.org/10.1016/S1054-3589(08)61078-9
Mourgeon E, Levesque E, Duveau C, Law-Koune JD, Charbit B, Ternissien E, Coriat P, Rouby JJ (1997) Factors influencing indoor concentrations of nitric oxide in a Parisian intensive care unit. Am J Respir Crit Care Med. https://doi.org/10.1164/ajrccm.156.5.96-12012
Norman V, Keith CH (1965) Nitrogen oxides in tobacco smoke. Nature 205:915
Gianni S, Carroll RW, Kacmarek RM, Berra L (2021) Inhaled nitric oxide delivery systems for mechanically ventilated and nonintubated patients: a review. Respir Care 66:1021–1028. https://doi.org/10.4187/respcare.08856
Miller MR, Megson IL (2007) Recent developments in nitric oxide donor drugs. Br J Pharmacol 151:305–321. https://doi.org/10.1038/sj.bjp.0707224
Ignarro LJ, Napoli C, Loscalzo J (2002) Nitric oxide donors and cardiovascular agents modulating the bioactivity of nitric oxide. Circ Res 90:21–28. https://doi.org/10.1161/hh0102.102330
Stuehr DJ, Santolini J, Wang Z-Q, Wei C-C, Adak S (2004) Update on mechanism and catalytic regulation in the NO synthases. J Biol Chem 279:36167–36170. https://doi.org/10.1074/jbc.R400017200
Förstermann U, Münzel T (2006) Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation 113:1708–1714. https://doi.org/10.1161/CIRCULATIONAHA.105.602532
Dudzinski DM, Igarashi J, Greif D, Michel T (2006) The regulation and pharmacology of endothelial nitric oxide synthase. Annu Rev Pharmacol Toxicol 46:235–276. https://doi.org/10.1146/annurev.pharmtox.44.101802.121844
Bredt DS, Hwang PM, Snyder SH (1990) Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature. https://doi.org/10.1038/347768a0
Abu-Soud HM, Wang J, Rousseau DL, Fukuto JM, Ignarro LJ, Stuehr DJ (1995) Neuronal nitric oxide synthase self-inactivates by forming a ferrous- nitrosyl complex during aerobic catalysis. J Biol Chem. https://doi.org/10.1074/jbc.270.39.22997
Salerno JC (2008) Neuronal nitric oxide synthase: prototype for pulsed enzymology. FEBS Lett. https://doi.org/10.1016/j.febslet.2008.03.051
Costa ED, Rezende BA, Cortes SF, Lemos VS (2016) Neuronal nitric oxide synthase in vascular physiology and diseases. Front Physiol 7:206. https://doi.org/10.3389/fphys.2016.00206
Santolini J, Meade AL, Stuehr DJ (2001) Differences in three kinetic parameters underpin the unique catalytic profiles of nitric-oxide synthases I, II, and III. J Biol Chem. https://doi.org/10.1074/jbc.M108666200
Förstermann U, Kleinert H (1995) Nitric oxide synthase: expression and expressional control of the three isoforms. Naunyn-Schmiedeberg’s Arch Pharmacol. https://doi.org/10.1007/BF00172772
Bogdan C (2001) Nitric oxide and the immune response. Nat Immunol 2:907–916. https://doi.org/10.1038/ni1001-907
Nathan C (1997) Inducible nitric oxide synthase: what difference does it make? J Clin Invest 100:2417–2423
Zhao XJ, Wang L, Shiva S, Tejero J, Myerburg MM, Wang J, Frizzell S, Gladwin MT (2013) Mechanisms for cellular NO oxidation and nitrite formation in lung epithelial cells. Free Radical Biol Med. https://doi.org/10.1016/j.freeradbiomed.2013.04.031
Voraphani N, Gladwin MT, Contreras AU, Kaminski N, Tedrow JR, Milosevic J, Bleecker ER, Meyers DA, Ray A, Ray P, Erzurum SC, Busse WW, Zhao J, Trudeau JB, Wenzel SE (2014) An airway epithelial iNOS-DUOX2-thyroid peroxidase metabolome drives Th1/Th2 nitrative stress in human severe asthma. Mucosal Immunol. https://doi.org/10.1038/mi.2014.6
Kobayashi A, Hashimoto S, Kooguchi K, Kitamura Y, Onodera H, Urata Y, Ashihara T (1998) Expression of inducible nitric oxide synthase and inflammatory cytokines in alveolar macrophages of ARDS following sepsis. Chest. https://doi.org/10.1378/chest.113.6.1632
Gunnett CA, Lund DD, McDowell AK, Faraci FM, Heistad DD (2005) Mechanisms of inducible nitric oxide synthase-mediated vascular dysfunction. Arterioscler Thromb Vasc Biol. https://doi.org/10.1161/01.ATV.0000172626.00296.ba
Eguchi D, D’Uscio LV, Wambi C, Weiler D, Kovesdi I, O’Brien T, Katusic ZS (2002) Inhibitory effect of recombinant iNOS gene expression on vasomotor function of canine basilar artery. Am J Physiol Heart Circ Physiol. https://doi.org/10.1152/ajpheart.00415.2002
Ignarro LJ, Byrns RE, Buga GM, Wood KS (1987) Endothelium-derived relaxing factor from pulmonary artery and vein possesses pharmacologic and chemical properties identical to those of nitric oxide radical. Circ Res. https://doi.org/10.1161/01.RES.61.6.866
Palmer RMJ, Ferrige AG, Moncada S (1987) Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. https://doi.org/10.1038/327524a0
Derubertis FR, Craven PA (1976) Calcium-independent modulation of cyclic GMP and activation of guanylate cyclase by nitrosamines. Science. https://doi.org/10.1126/science.7837
Gruetter CA, Barry BK, McNamara DB, Gruetter DY, Kadowitz PJ, Ignarro L (1979) Relaxation of bovine coronary artery and activation of coronary arterial guanylate cyclase by nitric oxide, nitroprusside and a carcinogenic nitrosoamine. J Cyclic Nucleotide Res 5:211–224
Corson MA, James NL, Latta SE, Nerem RM, Berk BC, Harrison DG (1996) Phosphorylation of endothelial nitric oxide synthase in response to fluid shear stress. Circ Res. https://doi.org/10.1161/01.RES.79.5.984
Deanfield JE, Halcox JP, Rabelink TJ (2007) Endothelial function and dysfunction: testing and clinical relevance. Circulation 115:1285–1295. https://doi.org/10.1161/CIRCULATIONAHA.106.652859
Garg UC, Hassid A (1989) Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J Clin Investig. https://doi.org/10.1172/JCI114081
Radomski MW, Vallance P, Whitley G, Foxwell N, Moncada S (1993) Platelet adhesion to human vascular endothelium is modulated by constitutive and cytokine induced nitric oxide. Cardiovasc Res. https://doi.org/10.1093/cvr/27.7.1380
Ignarro LJ (1989) Biological actions and properties of endothelium-derived nitric oxide formed and released from artery and vein. Circ Res 65:1–21. https://doi.org/10.1161/01.res.65.1.1
Lefer AM (1997) Nitric oxide: nature’s naturally occurring leukocyte inhibitor. Circulation 95:553–554. https://doi.org/10.1161/01.cir.95.3.553
Gao F, Lucke-Wold BP, Li X, Logsdon AF, Xu LC, Xu S, LaPenna KB, Wang H, Talukder MAH, Siedlecki CA, Huber JD, Rosen CL, He P (2018) Reduction of endothelial nitric oxide increases the adhesiveness of constitutive endothelial membrane ICAM-1 through Src-mediated phosphorylation. Front Physiol. https://doi.org/10.3389/fphys.2017.01124
Moncada S, Erusalimsky JD (2002) Does nitric oxide modulate mitochondrial energy generation and apoptosis? Nat Rev Mol Cell Biol 3:214–220. https://doi.org/10.1038/nrm762
Ignarro LJ, Degnan JN, Baricos WH, Kadowitz PJ, Wolin MS (1982) Activation of purified guanylate cyclase by nitric oxide requires heme comparison of heme-deficient, heme-reconstituted and heme-containing forms of soluble enzyme from bovine lung. BBA General Subjects. https://doi.org/10.1016/0304-4165(82)90008-3
Stamler JS, Lamas S, Fang FC (2001) Nitrosylation. The prototypic redox-based signaling mechanism. Cell 106:675–683. https://doi.org/10.1016/s0092-8674(01)00495-0
Ghosh S, Karin M (2002) Missing pieces in the NF-kappaB puzzle. Cell 109(Suppl):S81-96. https://doi.org/10.1016/s0092-8674(02)00703-1
Stamler JS, Singel DJ, Loscalzo J (1992) Biochemistry of nitric oxide and its redox-activated forms. Science. https://doi.org/10.1126/science.1281928
Stamler JS, Simon DI, Osborne JA, Mullins ME, Jaraki O, Michel T, Singel DJ, Loscalzo J (1992) S-Nitrosylation of proteins with nitric oxide: synthesis and characterization of biologically active compounds. Proc Natl Acad Sci USA. https://doi.org/10.1073/pnas.89.1.444
Jia L, Bonaventura C, Bonaventura J, Stamler JS (1996) S-nitrosohaemoglobin: a dynamic activity of blood involved in vascular control. Nature 380:221–226. https://doi.org/10.1038/380221a0
Sellke FW, Myers PR, Bates JN, Harrison DG (1990) Influence of vessel size on the sensitivity of porcine coronary microvessels to nitroglycerin. Am J Physiol Heart Circ Physiol. https://doi.org/10.1152/ajpheart.1990.258.2.h515
Ross JM, Fairchild HM, Weldy J, Guyton AC (1962) Autoregulation of blood flow by oxygen lack. Am J Physiol. https://doi.org/10.1152/ajplegacy.1962.202.1.21
Hess DT, Matsumoto A, Kim S-O, Marshall HE, Stamler JS (2005) Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol 6:150–166. https://doi.org/10.1038/nrm1569
Zhou H-L, Zhang R, Anand P, Stomberski CT, Qian Z, Hausladen A, Wang L, Rhee EP, Parikh SM, Karumanchi SA, Stamler JS (2019) Metabolic reprogramming by the S-nitroso-CoA reductase system protects against kidney injury. Nature 565:96–100. https://doi.org/10.1038/s41586-018-0749-z
Seth P, Hsieh PN, Jamal S, Wang L, Gygi SP, Jain MK, Coller J, Stamler JS (2019) Regulation of MicroRNA machinery and development by interspecies S-nitrosylation. Cell 176:1014-1025.e12. https://doi.org/10.1016/j.cell.2019.01.037
Premont RT, Reynolds JD, Zhang R, Stamler JS (2020) Role of nitric oxide carried by hemoglobin in cardiovascular physiology: developments on a three-gas respiratory cycle. Circ Res 126:129
Hottinger DG, Beebe DS, Kozhimannil T, Prielipp RC, Belani KG (2014) Sodium nitroprusside in 2014: a clinical concepts review. J Anaesthesiol Clin Pharmacol 30:462–471. https://doi.org/10.4103/0970-9185.142799
Chen ZQ, Mou RT, Feng DX, Wang Z, Chen G (2017) The role of nitric oxide in stroke. Med Gas Res. https://doi.org/10.4103/2045-9912.215750
Zhao X, Haensel C, Araki E, Ross ME, Iadecola C (2000) Gene-dosing effect and persistence of reduction in ischemic brain injury in mice lacking inducible nitric oxide synthase. Brain Res. https://doi.org/10.1016/S0006-8993(00)02459-8
Zhang F, Iadecola C (1994) Reduction of focal cerebral ischemic damage by delayed treatment with nitric oxide donors. J Cereb Blood Flow Metab. https://doi.org/10.1038/jcbfm.1994.71
Weinberger B, Laskin DL, Heck DE, Laskin JD (2001) The toxicology of inhaled nitric oxide. Toxicol Sci 59:5–16. https://doi.org/10.1093/toxsci/59.1.5
Hamad AM, Johnson SR, Knox AJ (1999) Antiproliferative effects of NO and ANP in cultured human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. https://doi.org/10.1152/ajplung.1999.277.5.l910
Yu SM, Hung LM, Lin CC (1997) cGMP-elevating agents suppress proliferation of vascular smooth muscle cells by inhibiting the activation of epidermal growth factor signaling pathway. Circulation. https://doi.org/10.1161/01.CIR.95.5.1269
Isomura K, Chikahira M, Teranishi K, Hamada K (1984) Induction of mutations and chromosome aberrations in lung cells following in vivo exposure of rats to nitrogen oxides. Mutation Res/Genetic Toxicol. https://doi.org/10.1016/0165-1218(84)90153-8
Nguyen T, Brunson D, Crespi CL, Penman BW, Wishnok JS, Tannenbaum SR (1992) DNA damage and mutation in human cells exposed to nitric oxide in vitro. Proc Natl Acad Sci USA. https://doi.org/10.1073/pnas.89.7.3030
Koppenol WH, Moreno JJ, Pryor WA, Ischiropoulos H, Beckman JS (1992) Peroxynitrite, a cloaked oxidant formed by nitric oxide and superoxide. Chem Res Toxicol. https://doi.org/10.1021/tx00030a017
Grisham MB, Jourd’Heuil D, Wink DA (1999) Nitric oxide. I. Physiological chemistry of nitric oxide and its metabolites:implications in inflammation. Am J Physiol 276:G315-321. https://doi.org/10.1152/ajpgi.1999.276.2.G315
Liaudet L, Soriano FG, Szabó C (2000) Biology of nitric oxide signaling. Crit Care Med 28:N37-52. https://doi.org/10.1097/00003246-200004001-00005
Yermilov V, Rubio J, Ohshima H (1995) Formation of 8-nitroguanine in DNA treated with peroxynitrite in vitro and its rapid removal from DNA by depurination. FEBS Lett. https://doi.org/10.1016/0014-5793(95)01281-6
Szabó C, Ohshima H (1997) DNA damage induced by peroxynitrite: subsequent biological effects. Nitric Oxide 1:373–385. https://doi.org/10.1006/niox.1997.0143
Müller B, Schäfer H, Barth P, von Wichert P (1994) Lung surfactant components in bronchoalveolar lavage after inhalation of NO2 as markers of altered surfactant metabolism. Lung. https://doi.org/10.1007/BF00185077
Matalon S, DeMarco V, Haddad IY, Myles C, Skimming JW, Schürch S, Cheng S, Cassin S (1996) Inhaled nitric oxide injures the pulmonary surfactant system of lambs in vivo. Am J Physiol Lung Cell Mol Physiol. https://doi.org/10.1152/ajplung.1996.270.2.l273
Hallman M, Waffarn F, Bry K, Turbow R, Kleinman MT, Mautz WJ, Rasmussen RE, Bhalla DK, Phalen RF (1996) Surfactant dysfunction after inhalation of nitric oxide. J Appl Physiol. https://doi.org/10.1152/jappl.1996.80.6.2026
Quezado ZMN, Eichacker PQ (2000) Inhaled nitric oxide: more than a selective pulmonary vasodilator. Crit Care Med 28:1235
Keaney JF, Simon DI, Stamler JS, Jaraki O, Scharfstein J, Vita JA, Loscalzo J (1993) NO forms an adduct with serum albumin that has endothelium-derived relaxing factor-like properties. J Clin Investig. https://doi.org/10.1172/JCI116364
Schini-Kerth VB (1999) Vascular biosynthesis of nitric oxide: effect on hemostasis and fibrinolysis. Transfus Clin Biol 6:355–363. https://doi.org/10.1016/s1246-7820(00)88980-6
Kermarrec N, Zunic P, Beloucif S, Benessiano J, Drouet L, Payen D (1998) Impact of inhaled nitric oxide on platelet aggregation and fibrinolysis in rats with endotoxic lung injury: role of cyclic guanosine 5’-monophosphate. Am J Respir Crit Care Med. https://doi.org/10.1164/ajrccm.158.3.9709097
Finer NN, Barrington KJ (2000) Nitric oxide therapy for the newborn infant. Semin Perinatol. https://doi.org/10.1016/S0146-0005(00)80058-0
Roberts JD, Fineman JR, Morin FC, Shaul PW, Rimar S, Schreiber MD, Polin RA, Zwass MS, Zayek MM, Gross I, Heymann MA, Zapol WM (1997) Inhaled nitric oxide and persistent pulmonary hypertension of the newborn. The Inhaled Nitric Oxide Study Group. N Engl J Med 336:605–610. https://doi.org/10.1056/NEJM199702273360902
Maeda N, Imaizumi K, Kon K, Shiga T (1987) A kinetic study on functional impairment of nitric oxide-exposed rat erythrocytes. Environ Health Perspect. https://doi.org/10.1289/ehp.8773171
Sharrock P, Lévy P, Massol M (1984) Blood analysis of rabbits exposed to nitrogen monoxide. Chemosphere. https://doi.org/10.1016/0045-6535(84)90170-X
Flammer AJ, Lüscher TF (2010) Human endothelial dysfunction: EDRFs. Pflugers Arch 459:1005–1013. https://doi.org/10.1007/s00424-010-0822-4
Virdis A, Ghiadoni L, Taddei S (2010) Human endothelial dysfunction: EDCFs. Pflugers Arch 459:1015–1023. https://doi.org/10.1007/s00424-009-0783-7
Tejero J, Shiva S, Gladwin MT (2019) Sources of vascular nitric oxide and reactive oxygen species and their regulation. Physiol Rev 99:311–379. https://doi.org/10.1152/physrev.00036.2017
Egea J, Fabregat I, Frapart YM, Ghezzi P, Görlach A, Kietzmann T, Kubaichuk K, Knaus UG, Lopez MG, Olaso-Gonzalez G, Petry A, Schulz R, Vina J, Winyard P, Abbas K, Ademowo OS, Afonso CB, Andreadou I, Antelmann H, Antunes F, Aslan M, Bachschmid MM, Barbosa RM, Belousov V, Berndt C, Bernlohr D, Bertrán E, Bindoli A, Bottari SP, Brito PM, Carrara G, Casas AI, Chatzi A, Chondrogianni N, Conrad M, Cooke MS, Costa JG, Cuadrado A, My-Chan Dang P, De Smet B, Debelec-Butuner B, Dias IHK, Dunn JD, Edson AJ, El Assar M, El-Benna J, Ferdinandy P, Fernandes AS, Fladmark KE, Förstermann U, Giniatullin R, Giricz Z, Görbe A, Griffiths H, Hampl V, Hanf A, Herget J, Hernansanz-Agustín P, Hillion M, Huang J, Ilikay S, Jansen-Dürr P, Jaquet V, Joles JA, Kalyanaraman B, Kaminskyy D, Karbaschi M, Kleanthous M, Klotz L-O, Korac B, Korkmaz KS, Koziel R, Kračun D, Krause K-H, Křen V, Krieg T, Laranjinha J, Lazou A, Li H, Martínez-Ruiz A, Matsui R, McBean GJ, Meredith SP, Messens J, Miguel V, Mikhed Y, Milisav I, Milković L, Miranda-Vizuete A, Mojović M, Monsalve M, Mouthuy P-A, Mulvey J, Münzel T, Muzykantov V, Nguyen ITN, Oelze M, Oliveira NG, Palmeira CM, Papaevgeniou N, Pavićević A, Pedre B, Peyrot F, Phylactides M, Pircalabioru GG, Pitt AR, Poulsen HE, Prieto I, Rigobello MP, Robledinos-Antón N, Rodríguez-Mañas L, Rolo AP, Rousset F, Ruskovska T, Saraiva N, Sasson S, Schröder K, Semen K, Seredenina T, Shakirzyanova A, Smith GL, Soldati T, Sousa BC, Spickett CM, Stancic A, Stasia MJ, Steinbrenner H, Stepanić V, Steven S, Tokatlidis K, Tuncay E, Turan B, Ursini F, Vacek J, Vajnerova O, Valentová K, Van Breusegem F, Varisli L, Veal EA, Yalçın AS, Yelisyeyeva O, Žarković N, Zatloukalová M, Zielonka J, Touyz RM, Papapetropoulos A, Grune T, Lamas S, Schmidt HHHW, Di Lisa F, Daiber A (2017) European contribution to the study of ROS: a summary of the findings and prospects for the future from the COST action BM1203 (EU-ROS). Redox Biol 13:94–162. https://doi.org/10.1016/j.redox.2017.05.007
Böger RH (2004) Asymmetric dimethylarginine, an endogenous inhibitor of nitric oxide synthase, explains the “L-arginine paradox” and acts as a novel cardiovascular risk factor. J Nutr 134:2842S-2847S. https://doi.org/10.1093/jn/134.10.2842S
Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, Fishman MC (1995) Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. https://doi.org/10.1038/377239a0
Lee PC, Salyapongse AN, Bragdon GA, Shears LL, Watkins SC, Edington HDJ, Billiar TR (1999) Impaired wound healing and angiogenesis in eNOS-deficient mice. Am J Physiol Heart Circ Physiol. https://doi.org/10.1152/ajpheart.1999.277.4.h1600
Nakayama M, Yasue H, Yoshimura M, Shimasaki Y, Kugiyama K, Ogawa H, Motoyama T, Saito Y, Ogawa Y, Miyamoto Y, Nakao K (1999) T-786 → C mutation in the 5’-flanking region of the endothelial nitric oxide synthase gene is associated with coronary spasm. Circulation. https://doi.org/10.1161/01.CIR.99.22.2864
Niu W, Qi Y (2011) An updated meta-analysis of endothelial nitric oxide synthase gene: three well-characterized polymorphisms with hypertension. PLoS ONE. https://doi.org/10.1371/journal.pone.0024266
Dai B, Liu T, Zhang B, Zhang X, Wang Z (2013) The polymorphism for endothelial nitric oxide synthase gene, the level of nitric oxide and the risk for pre-eclampsia: a meta-analysis. Gene. https://doi.org/10.1016/j.gene.2013.01.004
Shoukry A, Shalaby SM, Abdelazim S, Abdelazim M, Ramadan A, Ismail MI, Fouad M (2012) Endothelial nitric oxide synthase gene polymorphisms and the risk of diabetic nephropathy in type 2 diabetes mellitus. Genet Test Mol Biomarkers. https://doi.org/10.1089/gtmb.2011.0218
Taverna MJ, Elgrably F, Selmi H, Selam JL, Slama G (2005) The T-786C and C774T endothelial nitric oxide synthase gene polymorphisms independently affect the onset pattern of severe diabetic retinopathy. Nitric Oxide Biol Chem. https://doi.org/10.1016/j.niox.2005.04.004
Safarinejad MR, Khoshdel A, Shekarchi B, Taghva A, Safarinejad S (2011) Association of the T-786C, G894T and 4a/4b polymorphisms of the endothelial nitric oxide synthase gene with vasculogenic erectile dysfunction in Iranian subjects. BJU Int. https://doi.org/10.1111/j.1464-410X.2010.09755.x
Vita JA (2011) Endothelial function. Circulation 124:e906–e912. https://doi.org/10.1161/CIRCULATIONAHA.111.078824
Flammer AJ, Anderson T, Celermajer DS, Creager MA, Deanfield J, Ganz P, Hamburg NM, Lüscher TF, Shechter M, Taddei S, Vita JA, Lerman A (2012) The assessment of endothelial function: from research into clinical practice. Circulation. https://doi.org/10.1161/CIRCULATIONAHA.112.093245
Wessel DL, Adatia I, Giglia TM, Thompson JE, Kulik TJ (1993) Use of inhaled nitric oxide and acetylcholine in the evaluation of pulmonary hypertension and endothelial function after cardiopulmonary bypass. Circulation. https://doi.org/10.1161/01.CIR.88.5.2128
Hampl V, Cornfield DN, Cowan NJ, Archer SL (1995) Hypoxia potentiates nitric oxide synthesis and transiently increases cytosolic calcium levels in pulmonary artery endothelial cells. Eur Respir J 8:515–522
Blitzer ML, Lee SD, Creager MA (1996) Endothelium-derived nitric oxide mediates hypoxic vasodilation of resistance vessels in humans. Am J Physiol Heart Circ Physiol. https://doi.org/10.1152/ajpheart.1996.271.3.h1182
Blitzer ML, Loh E, Roddy M-A, Stamler JS, Creager MA (1996) Endothelium-derived nitric oxide regulates systemic and pulmonary vascular resistance during acute hypoxia in humans. J Am Coll Cardiol. https://doi.org/10.1016/0735-1097(96)00218-5
Zhang R, Hess DT, Qian Z, Hausladen A, Fonseca F, Chaube R, Reynolds JD, Stamler JS (2015) Hemoglobin βCys93 is essential for cardiovascular function and integrated response to hypoxia. Proc Natl Acad Sci USA. https://doi.org/10.1073/pnas.1502285112
Zhang R, Hess DT, Reynolds JD, Stamler JS (2016) Hemoglobin S-nitrosylation plays an essential role in cardioprotection. J Clin Investig. https://doi.org/10.1172/JCI90425
Nagasaka Y, Fernandez BO, Steinbicker AU, Spagnolli E, Malhotra R, Bloch DB, Bloch KD, Zapol WM, Feelisch M (2018) Pharmacological preconditioning with inhaled nitric oxide (NO): organ-specific differences in the lifetime of blood and tissue NO metabolites. Nitric Oxide Biol Chem. https://doi.org/10.1016/j.niox.2018.08.006
Ma XI, Weyrich AS, Lefer DJ, Lefer AM (1993) Diminished basal nitric oxide release after myocardial ischemia and reperfusion promotes neutrophil adherence to coronary endothelium. Circ Res. https://doi.org/10.1161/01.res.72.2.403
Guery B, Neviere R, Viget N, Foucher C, Fialdes P, Wattel F, Beaucaire G (1999) Inhaled NO preadministration modulates local and remote ischemia- reperfusion organ injury in a rat model. J Appl Physiol. https://doi.org/10.1152/jappl.1999.87.1.47
Dong BM, Abano JB, Egan TM (2009) Nitric oxide ventilation of rat lungs from non-heart-beating donors improves posttransplant function. Am J Transplant. https://doi.org/10.1111/j.1600-6143.2009.02840.x
Fox-Robichaud A, Payne D, Hasan SU, Ostrovsky L, Fairhead T, Reinhardt P, Kubes P (1998) Inhaled NO as a viable antiadhesive therapy for ischemia/reperfusion injury of distal microvascular beds. J Clin Investig. https://doi.org/10.1172/JCI2736
Siriussawakul A, Zaky A, Lang JD (2010) Role of nitric oxide in hepatic ischemia-reperfusion injury. World J Gastroenterol 16:6079–6086. https://doi.org/10.3748/wjg.v16.i48.6079
Mathru M, Huda R, Solanki DR, Hays S, Lang JD (2007) Inhaled nitric oxide attenuates reperfusion inflammatory responses in humans. Anesthesiology. https://doi.org/10.1097/00000542-200702000-00015
Lang JD, Teng X, Chumley P, Crawford JH, Isbell TS, Chacko BK, Liu Y, Jhala N, Crowe DR, Smith AB, Cross RC, Frenette L, Kelley EE, Wilhite DW, Hall CR, Page GP, Fallon MB, Bynon JS, Eckhoff DE, Patel RP (2007) Inhaled NO accelerates restoration of liver function in adults following orthotopic liver transplantation. J Clin Investig. https://doi.org/10.1172/JCI31892
Garry PS, Ezra M, Rowland MJ, Westbrook J, Pattinson KTS (2015) The role of the nitric oxide pathway in brain injury and its treatment–from bench to bedside. Exp Neurol 263:235–243. https://doi.org/10.1016/j.expneurol.2014.10.017
Huang Z, Huang PL, Ma J, Meng W, Ayata C, Fishman MC, Moskowitz MA (1996) Enlarged infarcts in endothelial nitric oxide synthase knockout mice are attenuated by nitro-L-arginine. J Cereb Blood Flow Metab. https://doi.org/10.1097/00004647-199609000-00023
Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA (2010) Glial and neuronal control of brain blood flow. Nature 468:232–243. https://doi.org/10.1038/nature09613
Stefanovic B, Schwindt W, Hoehn M, Silva AC (2007) Functional uncoupling of hemodynamic from neuronal response by inhibition of neuronal nitric oxide synthase. J Cereb Blood Flow Metab. https://doi.org/10.1038/sj.jcbfm.9600377
Thiel VE, Audus KL (2001) Nitric oxide and blood-brain barrier integrity. Antioxid Redox Signal 3:273–278. https://doi.org/10.1089/152308601300185223
Hirvonen MR, Brüne B, Lapetina EG (1996) Heat shock proteins and macrophage resistance to the toxic effects of nitric oxide. Biochem J. https://doi.org/10.1042/bj3150845
Sims NR, Anderson MF (2002) Mitochondrial contributions to tissue damage in stroke. Neurochem Int. https://doi.org/10.1016/S0197-0186(01)00122-X
Li YS, Shemmer B, Stone E, Nardi MA, Jonas S, Quartermain D (2013) Neuroprotection by inhaled nitric oxide in a murine stroke model is concentration and duration dependent. Brain Res. https://doi.org/10.1016/j.brainres.2013.02.031
Charriaut-Marlangue C, Bonnin P, Gharib A, Leger PL, Villapol S, Pocard M, Gressens P, Renolleau S, Baud O (2012) Inhaled nitric oxide reduces brain damage by collateral recruitment in a neonatal stroke model. Stroke. https://doi.org/10.1161/STROKEAHA.112.664243
Terpolilli NA, Kim SW, Thal SC, Kataoka H, Zeisig V, Nitzsche B, Klaesner B, Zhu C, Schwarzmaier S, Meissner L, Mamrak U, Engel DC, Drzezga A, Patel RP, Blomgren K, Barthel H, Boltze J, Kuebler WM, Plesnila N (2012) Inhalation of nitric oxide prevents ischemic brain damage in experimental stroke by selective dilatation of collateral arterioles. Circ Res. https://doi.org/10.1161/CIRCRESAHA.111.253419
Pluta RM (2008) Dysfunction of nitric oxide synthases as a cause and therapeutic target in delayed cerebral vasospasm after SAH. Acta Neurochir Suppl 104:139–147. https://doi.org/10.1007/978-3-211-75718-5_28
Hugelshofer M, Sikorski CM, Seule M, Deuel J, Muroi CI, Seboek M, Akeret K, Buzzi R, Regli L, Schaer DJ, Keller E (2018) Cell-free oxyhemoglobin in cerebrospinal fluid after aneurysmal subarachnoid hemorrhage: biomarker and potential therapeutic target. World Neurosurg. https://doi.org/10.1016/j.wneu.2018.08.141
Durmaz R, Ozkara E, Kanbak G, Arslan OC, Dokumacioğlu A, Kartkaya K, Atasoy MA (2008) Nitric oxide level and adenosine deaminase activity in cerebrospinal fluid of patients with subarachnoid hemorrhage. Turk Neurosurg 18:157–164
Pluta R, Oldfield E (2008) Analysis of nitric oxide (NO) in cerebral vasospasm after aneursymal bleeding. Rev Recent Clin Trials. https://doi.org/10.2174/157488707779318062
Sehba FA, Schwartz AY, Chereshnev I, Bederson JB (2000) Acute decrease in cerebral nitric oxide levels after subarachnoid hemorrhage. J Cereb Blood Flow Metab. https://doi.org/10.1097/00004647-200003000-00018
Ito Y, Isotani E, Mizuno Y, Azuma H, Hirakawa K (2000) Effective improvement of the cerebral vasospasm after subarachnoid hemorrhage with low-dose nitroglycerin. J Cardiovasc Pharmacol. https://doi.org/10.1097/00005344-200001000-00006
Egemen N, Turker RK, Sanlidilek U, Zorlutuna A, Bilgic S, Baskaya M, Unlu A, Caglar S, Spetzler RF, McCormick JM (1993) The effect of intrathecal sodium nitroprusside on severe chronic vasospasm. Neurol Res. https://doi.org/10.1080/01616412.1993.11740153
Hugelshofer M, Buzzi RM, Schaer CA, Richter H, Akeret K, Anagnostakou V, Mahmoudi L, Vaccani R, Vallelian F, Deuel JW, Kronen PW, Kulcsar Z, Regli L, Baek JH, Pires IS, Palmer AF, Dennler M, Humar R, Buehler PW, Kircher PR, Keller E, Schaer DJ (2019) Haptoglobin administration into the subarachnoid space prevents hemoglobin-induced cerebral vasospasm. J Clin Investig. https://doi.org/10.1172/JCI130630
Uhl E, Lehmberg J, Steiger HJ, Messmer K, Dempsey RJ, Stieg PE, Batjer HH, Steinmeier R, Schackert G, Solomon RA (2003) Intraoperative detection of early microvasospasm in patients with subarachnoid hemorrhage by using orthogonal polarization spectral imaging. Neurosurgery. https://doi.org/10.1227/01.NEU.0000065154.04824.9E
Pennings FA, Bouma GJ, Ince C (2004) Direct observation of the human cerebral microcirculation during aneurysm surgery reveals increased arteriolar contractility. Stroke. https://doi.org/10.1161/01.STR.0000126039.91400.cb
Friedrich B, Müller F, Feiler S, Schöller K, Plesnila N (2012) Experimental subarachnoid hemorrhage causes early and long-lasting microarterial constriction and microthrombosis: an in-vivo microscopy study. J Cereb Blood Flow Metab. https://doi.org/10.1038/jcbfm.2011.154
Terpolilli NA, Feiler S, Dienel A, Müller F, Heumos N, Friedrich B, Stover J, Thal S, Schöller K, Plesnila N (2016) Nitric oxide inhalation reduces brain damage, prevents mortality, and improves neurological outcome after subarachnoid hemorrhage by resolving early pial microvasospasms. J Cereb Blood Flow Metab. https://doi.org/10.1177/0271678X15605848
Abboud A, Mi Q, Puccio A, Okonkwo D, Buliga M, Constantine G, Vodovotz Y (2016) Inflammation following traumatic brain injury in humans: insights from data-driven and mechanistic models into survival and death. Front Pharmacol. https://doi.org/10.3389/fphar.2016.00342
Üçal M, Kraitsy K, Weidinger A, Paier-Pourani J, Patz S, Fink B, Molcanyi M, Schäfer U (2017) Comprehensive profiling of modulation of nitric oxide levels and mitochondrial activity in the injured brain: an experimental study based on the fluid percussion injury model in rats. J Neurotrauma. https://doi.org/10.1089/neu.2016.4411
Olmos G, Lladó J (2014) Tumor necrosis factor alpha: a link between neuroinflammation and excitotoxicity. Mediators Inflamm 2014:861231. https://doi.org/10.1155/2014/861231
Villalba N, Sackheim AM, Nunez IA, Hill-Eubanks DC, Nelson MT, Wellman GC, Freeman K (2017) Traumatic Brain injury causes endothelial dysfunction in the systemic microcirculation through arginase-1-dependent uncoupling of endothelial nitric oxide synthase. J Neurotrauma. https://doi.org/10.1089/neu.2015.4340
Bayir H, Kagan VE, Borisenko GG, Tyurina YY, Janesko KL, Vagni VA, Billiar TR, Williams DL, Kochanek PM (2005) Enhanced oxidative stress in iNOS-deficient mice after traumatic brain injury: support for a neuroprotective role of iNOS. J Cereb Blood Flow Metab. https://doi.org/10.1038/sj.jcbfm.9600068
Terpolilli NA, Kim SW, Thal SC, Kuebler WM, Plesnila N (2013) Inhaled nitric oxide reduces secondary brain damage after traumatic brain injury in mice. J Cereb Blood Flow Metab. https://doi.org/10.1038/jcbfm.2012.176
Hekierski H, Pastor P, Curvello V, Armstead WM (2019) Inhaled nitric oxide protects cerebral autoregulation and reduces hippocampal neuronal cell necrosis after traumatic brain injury in newborn and juvenile pigs. J Neurotrauma. https://doi.org/10.1089/neu.2018.5824
Curvello V, Pastor P, Hekierski H, Armstead WM (2019) Inhaled nitric oxide protects cerebral autoregulation and reduces hippocampal necrosis after traumatic brain injury through inhibition of ET-1, ERK MAPK and IL-6 upregulation in pigs. Neurocrit Care. https://doi.org/10.1007/s12028-018-0638-1
Miyazaki Y, Ichinose F (2020) Nitric oxide in post-cardiac arrest syndrome. J Cardiovasc Pharmacol 75:508–515. https://doi.org/10.1097/FJC.0000000000000765
Minamishima S, Kida K, Tokuda K, Wang H, Sips PY, Kosugi S, Mandeville JB, Buys ES, Brouckaert P, Liu PK, Liu CH, Bloch KD, Ichinose F (2011) Inhaled nitric oxide improves outcomes after successful cardiopulmonary resuscitation in mice. Circulation. https://doi.org/10.1161/CIRCULATIONAHA.111.025395
Hayashida K, Bagchi A, Miyazaki Y, Hirai S, Seth D, Silverman MG, Rezoagli E, Marutani E, Mori N, Magliocca A, Liu X, Berra L, Hindle AG, Donnino MW, Malhotra R, Bradley MO, Stamler JS, Ichinose F (2019) Improvement in outcomes after cardiac arrest and resuscitation by inhibition of S-nitrosoglutathione reductase. Circulation. https://doi.org/10.1161/CIRCULATIONAHA.117.032488
Patel JK, Schoenfeld E, Hou W, Singer A, Rakowski E, Ahmad S, Patel R, Parikh PB, Smaldone G (2021) Inhaled nitric oxide in adults with in-hospital cardiac arrest: a feasibility study. Nitric Oxide Biol Chem. https://doi.org/10.1016/j.niox.2021.07.001
Magliocca A, Rezoagli E, Zani D, Manfredi M, De Giorgio D, Olivari D, Fumagalli F, Langer T, Avalli L, Grasselli G, Latini R, Pesenti A, Bellani G, Ristagno G (2021) Cardiopulmonary resuscitation-associated lung Edema (CRALE). A translational study. Am J Respir Crit Care Med 203:447–457. https://doi.org/10.1164/rccm.201912-2454OC
Rezoagli E, Magliocca A, Grieco DL, Bellani G, Ristagno G (2022) Impact of lung structure on airway opening index during mechanical versus manual chest compressions in a porcine model of cardiac arrest. Respir Physiol Neurobiol 296:103807. https://doi.org/10.1016/j.resp.2021.103807
Beloncle FM, Merdji H, Lesimple A, Pavlovsky B, Yvin E, Savary D, Mercat A, Meziani F, Richard J-C (2022) Gas exchange and respiratory mechanics after a cardiac arrest: a clinical description of cardiopulmonary resuscitation-associated lung edema. Am J Respir Crit Care Med. https://doi.org/10.1164/rccm.202111-2644LE
Lefer DJ, Nakanishi K, Johnston WE, Vinten-Johansen J (1993) Antineutrophil and myocardial protecting actions of a novel nitric oxide donor after acute myocardial ischemia and reperfusion in dogs. Circulation. https://doi.org/10.1161/01.CIR.88.5.2337
Jones SP, Girod WG, Palazzo AJ, Granger DN, Grisham MB, Jourd’heuil D, Huang PL, Lefer DJ (1999) Myocardial ischemia-reperfusion injury is exacerbated in absence of endothelial cell nitric oxide synthase. Am J Physiol Heart Circ Physiol. https://doi.org/10.1152/ajpheart.1999.276.5.h1567
Elrod JW, Greer JJM, Bryan NS, Langston W, Szot JF, Gebregzlabher H, Janssens S, Feelisch M, Lefer DJ (2006) Cardiomyocyte-specific overexpression of NO synthase-3 protects against myocardial ischemia-reperfusion injury. Arterioscler Thromb Vasc Biol. https://doi.org/10.1161/01.ATV.0000224324.52466.e6
Hataishi R, Rodrigues AC, Neilan TG, Morgan JG, Buys E, Shiva S, Tambouret R, Jassal DS, Raher MJ, Furutani E, Ichinose F, Gladwin MT, Rosenzweig A, Zapol WM, Picard MH, Bloch KD, Scherrer-Crosbie M (2006) Inhaled nitric oxide decreases infarction size and improves left ventricular function in a murine model of myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. https://doi.org/10.1152/ajpheart.01172.2005
Liu X, Huang Y, Pokreisz P, Vermeersch P, Marsboom G, Swinnen M, Verbeken E, Santos J, Pellens M, Gillijns H, Van de Werf F, Bloch KD, Janssens S (2007) Nitric oxide inhalation improves microvascular flow and decreases infarction size after myocardial ischemia and reperfusion. J Am Coll Cardiol. https://doi.org/10.1016/j.jacc.2007.04.069
Janssens SP, Bogaert J, Zalewski J, Toth A, Adriaenssens T, Belmans A, Bennett J, Claus P, Desmet W, Dubois C, Goetschalckx K, Sinnaeve P, Vandenberghe K, Vermeersch P, Lux A, Szelid Z, Durak M, Lech P, Zmudka K, Pokreisz P, Vranckx P, Merkely B, Bloch KD, Van De Werf F (2018) Nitric oxide for inhalation in ST-elevation myocardial infarction (NOMI): a multicentre, double-blind, randomized controlled trial. Eur Heart J. https://doi.org/10.1093/eurheartj/ehy232
Helms C, Kim-Shapiro DB (2013) Hemoglobin-mediated nitric oxide signaling. Free Radic Biol Med 61:464–472. https://doi.org/10.1016/j.freeradbiomed.2013.04.028
Doyle MP, Hoekstra JW (1981) Oxidation of nitrogen oxides by bound dioxygen in hemoproteins. J Inorg Biochem. https://doi.org/10.1016/S0162-0134(00)80291-3
Minneci PC, Deans KJ, Zhi H, Yuen PST, Star RA, Banks SM, Schechter AN, Natanson C, Gladwin MT, Solomon SB (2005) Hemolysis-associated endothelial dysfunction mediated by accelerated NO inactivation by decompartmentalized oxyhemoglobin. J Clin Investig. https://doi.org/10.1172/JCI25040
Schaer CA, Deuel JW, Schildknecht D, Mahmoudi L, Garcia-Rubio I, Owczarek C, Schauer S, Kissner R, Banerjee U, Palmer AF, Spahn DR, Irwin DC, Vallelian F, Buehler PW, Schaer DJ (2016) Haptoglobin preserves vascular nitric oxide signaling during hemolysis. Am J Respir Crit Care Med. https://doi.org/10.1164/rccm.201510-2058OC
Graw JA, Yu B, Rezoagli E, Warren HS, Buys ES, Bloch DB, Zapol WM (2017) Endothelial dysfunction inhibits the ability of haptoglobin to prevent hemoglobin-induced hypertension. Am J Physiol Heart Circ Physiol. https://doi.org/10.1152/ajpheart.00851.2016
Yu B, Raher MJ, Volpato GP, Bloch KD, Ichinose F, Zapol WM (2008) Inhaled nitric oxide enables artificial blood transfusion without hypertension. Circulation. https://doi.org/10.1161/CIRCULATIONAHA.107.729137
Yu B, Shahid M, Egorina EM, Sovershaev MA, Raher MJ, Lei C, Wu MX, Bloch KD, Zapol WM (2010) Endothelial dysfunction enhances vasoconstriction due to scavenging of nitric oxide by a hemoglobin-based oxygen carrier. Anesthesiology. https://doi.org/10.1097/ALN.0b013e3181cd7838
Gladwin MT, Sachdev V, Jison ML, Shizukuda Y, Plehn JF, Minter K, Brown B, Coles WA, Nichols JS, Ernst I, Hunter LA, Blackwelder WC, Schechter AN, Rodgers GP, Castro O, Ognibene FP (2004) Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med. https://doi.org/10.1056/nejmoa035477
Parent F, Bachir D, Inamo J, Lionnet F, Driss F, Loko G, Habibi A, Bennani S, Savale L, Adnot S, Maitre B, Yaïci A, Hajji L, O’Callaghan DS, Clerson P, Girot R, Galacteros F, Simonneau G (2011) A hemodynamic study of pulmonary hypertension in sickle cell disease. N Engl J Med. https://doi.org/10.1056/nejmoa1005565
McMahon TJ, Shan S, Riccio DA, Batchvarova M, Zhu H, Telen MJ, Zennadi R (2019) Nitric oxide loading reduces sickle red cell adhesion and vaso-occlusion in vivo. Blood Adv. https://doi.org/10.1182/bloodadvances.2019031633
Bunn HF, Nathan DG, Dover GJ, Hebbel RP, Platt OS, Rosse WF, Ware RE (2010) Pulmonary hypertension and nitric oxide depletion in sickle cell disease. Blood 116:687–692. https://doi.org/10.1182/blood-2010-02-268193
Sertório JT, Neto-Neves EM, Dias-Junior CA, Sousa-Santos O, Kiss T, Mühl D, Tanus-Santos JE (2013) Elevated plasma hemoglobin levels increase nitric oxide consumption in experimental and clinical acute pulmonary thromboembolism. Crit Care Med. https://doi.org/10.1097/CCM.0b013e31827c0b43
Schulze-Neick I, Li J, Penny DJ, Redington AN (2001) Pulmonary vascular resistance after cardiopulmonary bypass in infants: Effect on postoperative recovery. J Thorac Cardiovasc Surg. https://doi.org/10.1067/mtc.2001.113747
Kam PCA, Hines L, O’Connor E (1996) Effects of cardiopulmonary bypass on systemic vascular resistance. Perfusion. https://doi.org/10.1177/026765919601100408
Toikkanen V, Rinne T, Huhtala H, Laurikka J, Porkkala H, Tarkka M, Mennander A (2014) Cardiopulmonary bypass decreases pulmonary vascular resistance index after coronary artery bypass surgery. Scand J Clin Lab Invest. https://doi.org/10.3109/00365513.2013.856032
Vermeulen Windsant IC, Hanssen SJ, Buurman WA, Jacobs MJ (2011) Cardiovascular surgery and organ damage: time to reconsider the role of hemolysis. J Thorac Cardiovasc Surg 142:1–11. https://doi.org/10.1016/j.jtcvs.2011.02.012
Hu J, Rezoagli E, Zadek F, Bittner EA, Lei C, Berra L (2021) Free Hemoglobin ratio as a novel biomarker of acute kidney injury after on-pump cardiac surgery: secondary analysis of a randomized controlled trial. Anesth Analg. https://doi.org/10.1213/ANE.0000000000005381
Rezoagli E, Ichinose F, Strelow S, Roy N, Shelton K, Matsumine R, Chen L, Bittner EA, Bloch DB, Zapol WM, Berra L (2017) Pulmonary and systemic vascular resistances after cardiopulmonary bypass: role of hemolysis. J Cardiothorac Vasc Anesth. https://doi.org/10.1053/j.jvca.2016.06.009
Vermeulen Windsant IC, de Wit NCJ, Sertorio JTC, van Bijnen AA, Ganushchak YM, Heijmans JH, Tanus-Santos JE, Jacobs MJ, Maessen JG, Buurman WA (2014) Hemolysis during cardiac surgery is associated with increased intravascular nitric oxide consumption and perioperative kidney and intestinal tissue damage. Front Physiol. https://doi.org/10.3389/fphys.2014.00340
Lei C, Berra L, Rezoagli E, Yu B, Dong H, Yu S, Hou L, Chen M, Chen W, Wang H, Zheng Q, Shen J, Jin Z, Chen T, Zhao R, Christie E, Sabbisetti VS, Nordio F, Bonventre JV, Xiong L, Zapol WM (2018) Nitric oxide decreases acute kidney injury and stage 3 chronic kidney disease after cardiac surgery. Am J Respir Crit Care Med. https://doi.org/10.1164/rccm.201710-2150OC
Marrazzo F, Spina S, Zadek F, Lama T, Xu C, Larson G, Rezoagli E, Malhotra R, Zheng H, Bittner EA, Shelton K, MelnitchoUK S, Roy N, Sundt TM, Riley WD, Williams P, Fisher D, Kacmarek RM, Thompson TB, Bonventre J, Zapol W, Ichinose F, Berra L (2019) Protocol of a randomised controlled trial in cardiac surgical patients with endothelial dysfunction aimed to prevent postoperative acute kidney injury by administering nitric oxide gas. BMJ Open. https://doi.org/10.1136/bmjopen-2018-026848
Wang D, Sun J, Solomon SB, Klein HG, Natanson C (2012) Transfusion of older stored blood and risk of death: a meta-analysis. Transfusion. https://doi.org/10.1111/j.1537-2995.2011.03466.x
Koch CG, Li L, Sessler DI, Figueroa P, Hoeltge GA, Mihaljevic T, Blackstone EH (2008) Duration of red-cell storage and complications after cardiac surgery. N Engl J Med. https://doi.org/10.1056/nejmoa070403
Bennett-Guerrero E, Veldman TH, Doctor A, Telen MJ, Ortel TL, Reid TS, Mulherin MA, Zhu H, Buck RD, Califf RM, McMahon TJ (2007) Evolution of adverse changes in stored RBCs. Proc Natl Acad Sci USA. https://doi.org/10.1073/pnas.0708160104
Donadee C, Raat NJH, Kanias T, Tejero J, Lee JS, Kelley EE, Zhao X, Liu C, Reynolds H, Azarov I, Frizzell S, Meyer EM, Donnenberg AD, Qu L, Triulzi D, Kim-Shapiro DB, Gladwin MT (2011) Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation. https://doi.org/10.1161/CIRCULATIONAHA.110.008698
Berra L, Pinciroli R, Stowell CP, Wang L, Yu B, Fernandez BO, Feelisch M, Mietto C, Hod EA, Chipman D, Scherrer-Crosbie M, Bloch KD, Zapol WM (2014) Autologous transfusion of stored red blood cells increases pulmonary artery pressure. Am J Respir Crit Care Med. https://doi.org/10.1164/rccm.201405-0850OC
Baron DM, Yu B, Lei C, Bagchi A, Beloiartsev A, Stowell CP, Steinbicker AU, Malhotra R, Bloch KD, Zapol WM (2012) Pulmonary hypertension in lambs transfused with stored blood is prevented by breathing nitric oxide. Anesthesiology. https://doi.org/10.1097/ALN.0b013e318246ef77
Sengupta P, Mahalakshmi V, Stebin JJ, Ganesh S, Suganya N, Chatterjee S (2020) Nitric oxide donors offer protection to RBC from storage lesion. Transfus Clin Biol. https://doi.org/10.1016/j.tracli.2020.07.002
Muenster S, Beloiartsev A, Yu B, Du E, Abidi S, Dao M, Fabry G, Graw JA, Wepler M, Malhotra R, Fernandez BO, Feelisch M, Bloch KD, Bloch DB, Zapol WM (2016) Exposure of stored packed erythrocytes to nitric oxide prevents transfusion-associated pulmonary hypertension. Anesthesiology. https://doi.org/10.1097/ALN.0000000000001294
Yazer MH, Waters JH, Elkin KR, Rohrbaugh ME, Kameneva MV (2008) A comparison of hemolysis and red cell mechanical fragility in blood collected with different cell salvage suction devices. Transfusion. https://doi.org/10.1111/j.1537-2995.2008.01670.x
Nishiyama T, Hanaoka K (2000) Free hemoglobin concentrations in patients receiving massive blood transfusion during emergency surgery for trauma. Can J Anesth. https://doi.org/10.1007/BF03019668
Lotterman S, Sharma S (2022) Blood Transfusion. In: StatPearls. StatPearls Publishing, Treasure Island (FL)
Khan F, Singh K, Friedman MT (2020) Artificial blood: the history and current perspectives of blood substitutes. Discoveries. https://doi.org/10.15190/d.2020.1
Bradford JR, Dean HP (1894) The pulmonary circulation. J Physiol. https://doi.org/10.1113/jphysiol.1894.sp000493
Euler US, Liljestrand G (1946) Observations on the pulmonary arterial blood pressure in the cat. Acta Physiol Scand. https://doi.org/10.1111/j.1748-1716.1946.tb00389.x
Madden JA, Dawson CA, Harder DR (1985) Hypoxia-induced activation in small isolated pulmonary arteries from the cat. J Appl Physiol. https://doi.org/10.1152/jappl.1985.59.1.113
Madden JA, Vadula MS, Kurup VP (1992) Effects of hypoxia and other vasoactive agents on pulmonary and cerebral artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. https://doi.org/10.1152/ajplung.1992.263.3.l384
Yuan XJ, Tod ML, Rubin LJ, Blaustein MP (1990) Contrasting effects of hypoxia on tension in rat pulmonary and mesenteric arteries. Am J Physiol Heart Circ Physiol. https://doi.org/10.1152/ajpheart.1990.259.2.h281
Zapol WM (1987) Diving adaptations of the Weddell seal. Sci Am. https://doi.org/10.1038/scientificamerican0687-100
Zapol WM, Rie M, Frikker M, Snider M, Quinn D (1985) Pulmonary circulation during adult respiratory distress syndrome. In: Zapol W, Falke K (eds) Acute respiratory failure. Marcel Dekker, p 241
Frostell C, Fratacci MD, Wain JC, Jones R, Zapol WM (1991) Inhaled nitric oxide: a selective pulmonary vasodilator reversing hypoxic pulmonary vasoconstriction. Circulation. https://doi.org/10.1161/01.CIR.83.6.2038
Berra L, Rodriguez-Lopez J, Rezoagli E, Yu B, Fisher DF, Semigran MJ, Bloch DB, Channick RN, Zapol WM (2016) Electric plasma–generated nitric oxide: hemodynamic effects in patients with pulmonary hypertension. Am J Respir Crit Care Med 194:1168–1170. https://doi.org/10.1164/rccm.201604-0834LE
Vonbank K, Ziesche R, Higenbottam TW, Stiebellehner L, Petkov V, Schenk P, Germann P, Block LH (2003) Controlled prospective randomised trial on the effects on pulmonary haemodynamics of the ambulatory long term use of nitric oxide and oxygen in patients with severe COPD. Thorax. https://doi.org/10.1136/thorax.58.4.289
Troncy E, Collet JP, Shapiro S, Guimond JG, Blair L, Ducruet T, Francœur M, Charbonneau M, Blaise G (1998) Inhaled nitric oxide in acute respiratory distress syndrome: a pilot randomized controlled study. Am J Respir Crit Care Med. https://doi.org/10.1164/ajrccm.157.5.9707090
Michael JR, Barton RG, Saffle JR, Mone M, Markewitz BA, Hillier K, Elstad MR, Campbell EJ, Troyer BE, Whatley RE, Liou TG, Samuelson WM, Carveth HJ, Hinson DM, Morris SE, Davis BL, Day RW (1998) Inhaled nitric oxide versus conventional therapy: effect on oxygenation in ARDS. Am J Respir Crit Care Med. https://doi.org/10.1164/ajrccm.157.5.96-10089
Dellinger RP, Zimmerman JL, Taylor RW, Sträube RC, Hauser DL, Criner GJ, Davis K, Hyers TM, Papadakos P (1998) Effects of inhaled nitric oxide in patients with acute respiratory distress syndrome: results of a randomized phase II trial. Crit Care Med. https://doi.org/10.1097/00003246-199801000-00011
Taylor RW, Zimmerman JL, Dellinger RP, Straube RC, Criner GJ, Davis K, Kelly KM, Smith TC, Small RJ (2004) Low-dose inhaled nitric oxide in patients with acute lung injury: a randomized controlled trial. J Am Med Assoc. https://doi.org/10.1001/jama.291.13.1603
Rezoagli E, Fumagalli R, Bellani G (2017) Definition and epidemiology of acute respiratory distress syndrome. Ann Transl Med 5:282. https://doi.org/10.21037/atm.2017.06.62
Rezoagli E, Laffey JG, Bellani G (2022) Monitoring lung injury severity and ventilation intensity during mechanical ventilation. Semin Respir Crit Care Med
Roberts JD, Polaner DM, Zapol WM, Lang P (1992) Inhaled nitric oxide in persistent pulmonary hypertension of the newborn. The Lancet. https://doi.org/10.1016/0140-6736(92)92686-A
Kinsella JP, Neish SR, Shaffer E, Abman SH (1992) Low-dose inhalation nitric oxide in persistent pulmonary hypertension of the newborn. Lancet 340:819–820. https://doi.org/10.1016/0140-6736(92)92687-B
Wu HW, Li ZG, Liu G, Lu GZ, Liang HY (2016) Effect of nitric oxide inhalation for the treatment of neonatal pulmonary hypertension. Eur Rev Med Pharmacol Sci 20:4607–4611
Cressoni M, Caironi P, Polli F, Carlesso E, Chiumello D, Cadringher P, Quintel M, Ranieri VM, Bugedo G, Gattinoni L (2008) Anatomical and functional intrapulmonary shunt in acute respiratory distress syndrome. Crit Care Med. https://doi.org/10.1097/01.CCM.0000300276.12074.E1
Roissant R, Falke K, Lopez F, Slama K, Pison U, Zapol WM (1993) Inhaled nitric oxide for the adult respiratory distress syndrome. N Engl J Med 328:399–405
Kosutova P, Mikolka P, Balentova S, Adamkov M, Mokra D (2019) Effects of nitric oxide donor on the lung functions in a saline lavage-induced model of ARDS. Physiol Res. https://doi.org/10.33549/physiolres.934365
Jacobs BR, Brilli RJ, Ballard ET, Passerini DJ, Smith DJ (1998) Aerosolized soluble nitric oxide donor improves oxygenation and pulmonary hypertension in acute lung injury. Am J Respir Crit Care Med. https://doi.org/10.1164/ajrccm.158.5.9802114
Adhikari NKJ, Dellinger RP, Lundin S, Payen D, Vallet B, Gerlach H, Park KJ, Mehta S, Slutsky AS, Friedrich JO (2014) Inhaled nitric oxide does not reduce mortality in patients with acute respiratory distress syndrome regardless of severity: systematic review and meta-analysis. Crit Care Med 42:404–412. https://doi.org/10.1097/CCM.0b013e3182a27909
Griffiths MJD, Evans TW (2005) Inhaled nitric oxide therapy in adults. N Engl J Med 353:2683–2695. https://doi.org/10.1056/NEJMra051884
Duggal A, Rezoagli E, Pham T, McNicholas BA, Fan E, Bellani G, Rubenfeld G, Pesenti AM, Laffey JG, LUNG SAFE Investigators and the ESICM Trials Group (2020) Patterns of use of adjunctive therapies in patients with early moderate to severe ARDS: insights from the LUNG SAFE study. Chest 157:1497–1505. https://doi.org/10.1016/j.chest.2020.01.041
Fan E, Del Sorbo L, Goligher EC, Hodgson CL, Munshi L, Walkey AJ, Adhikari NKJ, Amato MBP, Branson R, Brower RG, Ferguson ND, Gajic O, Gattinoni L, Hess D, Mancebo J, Meade MO, McAuley DF, Pesenti A, Ranieri VM, Rubenfeld GD, Rubin E, Seckel M, Slutsky AS, Talmor D, Thompson BT, Wunsch H, Uleryk E, Brozek J, Brochard LJ, American Thoracic Society, European Society of Intensive Care Medicine, and Society of Critical Care Medicine (2017) An Official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine Clinical Practice Guideline: mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 195:1253–1263. https://doi.org/10.1164/rccm.201703-0548ST
Griffiths MJD, McAuley DF, Perkins GD, Barrett N, Blackwood B, Boyle A, Chee N, Connolly B, Dark P, Finney S, Salam A, Silversides J, Tarmey N, Wise MP, Baudouin SV (2019) Guidelines on the management of acute respiratory distress syndrome. BMJ Open Respir Res 6:e000420. https://doi.org/10.1136/bmjresp-2019-000420
Redaelli S, Magliocca A, Malhotra R, Ristagno G, Citerio G, Bellani G, Berra L, Rezoagli E (2022) Nitric oxide: clinical applications in critically ill patients. Nitric Oxide 121:20–33. https://doi.org/10.1016/j.niox.2022.01.007
Pelosi P, D’Onofrio D, Chiumello D, Paolo S, Chiara G, Capelozzi VL, Barbas CSV, Chiaranda M, Gattinoni L (2003) Pulmonary and extrapulmonary acute respiratory distress syndrome are different. Eur Respir J Suppl 42:48s–56s. https://doi.org/10.1183/09031936.03.00420803
Madotto F, Rezoagli E, McNicholas BA, Pham T, Slutsky AS, Bellani G, Laffey JG, LUNG SAFE Investigators and the ESICM Trials Group (2020) Patterns and impact of arterial CO2 management in patients with acute respiratory distress syndrome: insights from the LUNG SAFE study. Chest 158:1967–1982. https://doi.org/10.1016/j.chest.2020.05.605
Madotto F, Rezoagli E, Pham T, Schmidt M, McNicholas B, Protti A, Panwar R, Bellani G, Fan E, van Haren F, Brochard L, Laffey JG, LUNG SAFE Investigators and the ESICM Trials Group (2020) Hyperoxemia and excess oxygen use in early acute respiratory distress syndrome: insights from the LUNG SAFE study. Crit Care 24:125. https://doi.org/10.1186/s13054-020-2826-6
Rezoagli E, McNicholas BA, Madotto F, Pham T, Bellani G, Laffey JG, LUNG SAFE Investigators, the ESICM Trials Group (2022) Presence of comorbidities alters management and worsens outcome of patients with acute respiratory distress syndrome: insights from the LUNG SAFE study. Ann Intensive Care 12:42. https://doi.org/10.1186/s13613-022-01015-7
McNicholas BA, Rezoagli E, Pham T, Madotto F, Guiard E, Fanelli V, Bellani G, Griffin MD, Ranieri M, Laffey JG, ESICM Trials Group and the Large observational study to UNderstand the Global impact of Severe Acute respiratory FailurE (LUNG SAFE) Investigators (2019) Impact of early acute kidney injury on management and outcome in patients with acute respiratory distress syndrome: a secondary analysis of a multicenter observational study. Crit Care Med 47:1216–1225. https://doi.org/10.1097/CCM.0000000000003832
Rezoagli E, McNicholas B, Pham T, Bellani G, Laffey JG (2020) Lung-kidney cross-talk in the critically ill: insights from the Lung Safe study. Intensive Care Med 46:1072–1073. https://doi.org/10.1007/s00134-020-05962-2
McNicholas BA, Madotto F, Pham T, Rezoagli E, Masterson CH, Horie S, Bellani G, Brochard L, Laffey JG, LUNG SAFE Investigators and the ESICM Trials Group (2019) Demographics, management and outcome of females and males with acute respiratory distress syndrome in the LUNG SAFE prospective cohort study. Eur Respir J 54:1900609. https://doi.org/10.1183/13993003.00609-2019
Madotto F, McNicholas B, Rezoagli E, Pham T, Laffey JG, Bellani G, LUNG SAFE Investigators, ESICM Trials Group (2021) Death in hospital following ICU discharge: insights from the LUNG SAFE study. Crit Care 25:144. https://doi.org/10.1186/s13054-021-03465-0
Iotti GA, Olivei MC, Palo A, Galbusera C, Veronesi R, Braschi A (1998) Acute effects of inhaled nitric oxide in adult respiratory distress syndrome. Eur Respir J 12:1164–1171
Dose–Response Characteristics during Long-Term Inhalation of Nitric Oxide in Patients with Severe Acute Respiratory Distress Syndrome | A Prospective, Randomized, Controlled Study | American Journal of Respiratory and Critical Care Medicine. https://doi.org/10.1164/rccm.2108121. Accessed 26 May 2022
Maddali MV, Churpek M, Pham T, Rezoagli E, Zhuo H, Zhao W, He J, Delucchi KL, Wang C, Wickersham N, McNeil JB, Jauregui A, Ke S, Vessel K, Gomez A, Hendrickson CM, Kangelaris KN, Sarma A, Leligdowicz A, Liu KD, Matthay MA, Ware LB, Laffey JG, Bellani G, Calfee CS, Sinha P, LUNG SAFE Investigators and the ESICM Trials Group (2022) Validation and utility of ARDS subphenotypes identified by machine-learning models using clinical data: an observational, multicohort, retrospective analysis. Lancet Respir Med 10:367–377. https://doi.org/10.1016/S2213-2600(21)00461-6
Shah FA, Meyer NJ, Angus DC, Awdish R, Azoulay É, Calfee CS, Clermont G, Gordon AC, Kwizera A, Leligdowicz A, Marshall JC, Mikacenic C, Sinha P, Venkatesh B, Wong HR, Zampieri FG, Yende S (2021) A research agenda for precision medicine in sepsis and acute respiratory distress syndrome: an official American thoracic society research statement. Am J Respir Crit Care Med 204:891–901. https://doi.org/10.1164/rccm.202108-1908ST
Horie S, McNicholas B, Rezoagli E, Pham T, Curley G, McAuley D, O’Kane C, Nichol A, Dos Santos C, Rocco PRM, Bellani G, Laffey JG (2020) Emerging pharmacological therapies for ARDS: COVID-19 and beyond. Intensive Care Med 46:2265–2283. https://doi.org/10.1007/s00134-020-06141-z
Lambden S (2019) Bench to bedside review: therapeutic modulation of nitric oxide in sepsis—an update. Intensive Care Med Exp. https://doi.org/10.1186/s40635-019-0274-x
Croen KD (1993) Evidence for an antiviral effect of nitric oxide. Inhibition of herpes simplex virus type 1 replication. J Clin Invest. https://doi.org/10.1172/JCI116479
Klingström J, Åkerström S, Hardestam J, Stoltz M, Simon M, Falk KI, Mirazimi A, Rottenberg M, Lundkvist Å (2006) Nitric oxide and peroxynitrite have different antiviral effects against hantavirus replication and free mature virions. Eur J Immunol. https://doi.org/10.1002/eji.200535587
Nathan CF, Hibbs JB (1991) Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr Opin Immunol. https://doi.org/10.1016/0952-7915(91)90079-G
James SL, Glaven J (1989) Macrophage cytotoxicity against schistosomula of Schistosoma mansoni involves arginine-dependent production of reactive nitrogen intermediates. J Immunol 143:4208–4212
Denis M (1991) Interferon-gamma-treated murine macrophages inhibit growth of tubercle bacilli via the generation of reactive nitrogen intermediates. Cell Immunol. https://doi.org/10.1016/0008-8749(91)90014-3
Alspaugh JA, Granger DL (1991) Inhibition of Cryptococcus neoformans replication by nitrogen oxides supports the role of these molecules as effectors of macrophage-mediated cytostasis. Infect Immun. https://doi.org/10.1128/iai.59.7.2291-2296.1991
Keyaerts E, Vijgen L, Chen L, Maes P, Hedenstierna G, Van Ranst M (2004) Inhibition of SARS-coronavirus infection in vitro by S-nitroso-N-acetylpenicillamine, a nitric oxide donor compound. Int J Infect Dis 8:223–226. https://doi.org/10.1016/j.ijid.2004.04.012
Rezoagli E, Magliocca A, Bellani G, Pesenti A, Grasselli G (2021) Development of a critical care response—experiences from Italy during the coronavirus disease 2019 pandemic. Anesthesiol Clin 39:265–284. https://doi.org/10.1016/j.anclin.2021.02.003
Akaberi D, Krambrich J, Ling J, Luni C, Hedenstierna G, Järhult JD, Lennerstrand J, Lundkvist Å (2020) Mitigation of the replication of SARS-CoV-2 by nitric oxide in vitro. Redox Biol. https://doi.org/10.1016/j.redox.2020.101734
Ricciardolo FLM, Bertolini F, Carriero V, Högman M (2020) Nitric oxide’s physiologic effects and potential as a therapeutic agent against COVID-19. J Breath Res 15:014001. https://doi.org/10.1088/1752-7163/abc302
Acknowledgements
The authors of this review would like to dedicate this manuscript to the memory of Warren M. Zapol, M.D., a visionary scientist, a tireless mentor and a scientific father of generations of physicians. His discovery on the therapeutic use of inhaled nitric oxide gas is one of the major contributions he gave to the critical care fields which expanded tremendously since then and the focus of our present review.
Funding
Dr. Graw is supported by the Deutsche Forschungsgemeinschaft (DFG GR 4446/3-1). Dr. Malhotra is supported by a COVID Fast Grant (George Mason University), the National Heart, Lung, and Blood Institute (R01HL142809), and the Wild Family Foundation. LB receives salary support from K23 HL128882/NHLBI NIH as principal investigator for his work on hemolysis and nitric oxide. LB receives technologies and devices from iNO Therapeutics LLC, Praxair Inc., Masimo Corp. LB receives grants from “Fast Grants for COVID-19 research” at Mercatus Center of George Mason University and from iNO Therapeutics LLC. Laboratory work is supported by the Reginald Jenney Endowment Chair at Harvard Medical School, by Sundry Funds at Massachusetts General Hospital, and by laboratory funds of the Anesthesia Center for Critical Care Research of the Department of Anesthesia, Critical Care and Pain Medicine at Massachusetts General Hospital. Dr. Rezoagli is supported by the Bicocca Starting grant 2020 from the University of Milano-Bicocca with the project titled: “Functional Residual Capacity Assessment using a Wash-In/Wash-Out technique based on a fast main-stream O2 Sensor with nanofluorescenT geometry for severe lung injury (FAST)—COVID and beyond”; by the International Young Investigator Award 2018 from European Society of Intensive Care Medicine (ESICM) with the project titled: “Role of the exhaled breath condensate as non-invasive monitoring of the lung inflammation during ARDS: a prospective cohort study”; and by the National Merck Sharp & Dohme Corporation Research Award 2017 from the Società Italiana di Anestesia Analgesia Rianimazione e Terapia Intensiva (SIAARTI) with the project titled: “Studio della concentrazione di ossido nitrico nell’esalato espiratorio come marcatore di danno polmonare acuto in pazienti adulti con ARDS sottoposti a ventilazione meccanica”.
Author information
Authors and Affiliations
Contributions
DS searched literature and wrote the manuscript. AM, KH, JAG, RM, GB, and LB critically revised the manuscript for important intellectual content. ER conceived the study, searched literature and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors have no competing interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Signori, D., Magliocca, A., Hayashida, K. et al. Inhaled nitric oxide: role in the pathophysiology of cardio-cerebrovascular and respiratory diseases. ICMx 10, 28 (2022). https://doi.org/10.1186/s40635-022-00455-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40635-022-00455-6