Abstract
Purpose
To study the feasibility of predicting fluid responsiveness (FR) by passive leg raising (PLR) using a Bioreactance-based noninvasive cardiac output monitoring device (NICOM).
Method
This prospective, two-center study included 75 consecutive intensive care unit (ICU) adult patients immediately after cardiac surgery. NICOM was used to continuously record cardiac output (CO) at baseline, during a PLR, and then during a 500 ml i.v. rapid colloid infusion. We estimated the precision of NICOM at baseline to derive the least minimum significant change (LMSC) in CO. We studied the predictability of PLR for FR by systematic analysis of different categorizations of PLR and FR, based on percentage change in CO (from 0 to 20%).
Results
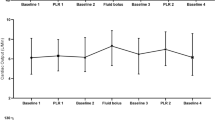
The LMSC was 8.85%. CO was 4.17 ± 1.04 L min−1 at baseline, 4.38 ± 1.14 L min−1 during PLR, 4.16 ± 1.08 L min−1 upon return to baseline, and 4.85 ± 1.41 L min−1 after fluid infusion. The change in CO following fluid bolus was highly correlated with the change in CO following PLR: y = 0.91x + 4.3, r = 0.77. The Pearson correlation coefficient showed that the best pair of thresholds was found for PLR ≥0% predicting FR ≥0%. Using this pair of thresholds, PLR had 88% sensitivity and 100% specificity for predicting FR. When we restricted the analysis to change in CO > LMSC, the best pair of thresholds was obtained for PLR >9% predicting FR >9%. Using this pair of thresholds, PLR sensitivity was reduced to 68% and specificity to 95%.
Conclusions
In this specific population of patients, it is clinically valid to use the bioreactance-based NICOM system to predict FR from changes in CO during PLR.



Similar content being viewed by others
References
Shoemaker W, Appel P, Kram H, Waxman K, Lee T (1988) Prospective trial of supranormal values of survivors as therapeutic goals in high-risk surgical patients. Chest 94:1176–1186
Boyd O, Grounds RM, Bennett ED (1993) A randomized clinical trial of the effect of deliberate perioperative increase of oxygen delivery on mortality in high-risk surgical patients. JAMA 270:2699–2707
Lobo SM, Lobo FR, Polachini CA, Patini DS, Yamamoto AE, de Oliveira NE, Serrano P, Sanches HS, Spegiorin MA, Queiroz MM, Christiano AC Jr, Savieiro EF, Alvarez PA, Teixeira SP, Cunrath GS (2006) Prospective, randomized trial comparing fluids and dobutamine optimization of oxygen delivery in high-risk surgical patients. Crit Care 10:R72
Joshi GP (2005) Intraoperative fluid restriction improves outcome after major elective gastrointestinal surgery. Anesth Analg 101:601–605
Martinez EA, Pronovost P (2002) Perioperative beta-blockers in high-risk patients. J Crit Care 17:105–113
Squara P, Dhainaut J, Lamy M, Perret C, Larbuisson R, Poli S, Armaganidis A, de Gournay J, Bleichner G (1989) Computer assistance for hemodynamic evaluation. J Crit Care 4:273–282
Chytra I, Pradl R, Bosman R, Pelnar P, Kasal E, Zidkova A (2007) Esophageal Doppler-guided fluid management decreases blood lactate levels in multiple-trauma patients: a randomized controlled trial. Crit Care 11:R24
Sinclair S, James S, Singer M (1997) Intraoperative intravascular volume optimisation and length of hospital stay after repair of proximal femoral fracture: randomised controlled trial. BMJ 315:909–912
Phan T, Ismail H, Heriot A, Ho K (2008) Improving perioperative outcomes: fluid optimization with the esophageal Doppler monitor, a metaanalysis and review. J Am Coll Surg 207:935–941
Stewart R, Park P, Hunt J, McIntyre RJ, McCarthy J, Zarzabal L, Michalek J (2009) Less is more: improved outcomes in surgical patients with conservative fluid administration and central venous catheter monitoring. J Am Coll Surg 208:725–735; discussion 735–727
Wiedemann H, Wheeler A, Bernard G, Thompson B, Hayden D, deBoisblanc B, Connors AJ, Hite R, Harabin A (2006) Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 354:2564–2575
Lafanechere A, Pene F, Goulenok C, Goulenok C, Delahaye A, Mallet V, Choukroun G, Chiche J, Mira J, Cariou A (2006) Changes in aortic blood flow induced by passive leg raising predict fluid responsiveness in critically ill patients. Crit Care 10:R132
Monnet X, Teboul J (2008) Passive leg raising. Intensive Care Med 34:659–663
Teboul J, Monnet X (2008) Prediction of volume responsiveness in critically ill patients with spontaneous breathing activity. Curr Opin Crit Care 14:334–339
Caille V, Jabot J, Belliard G, Charron C, Jardin F, Vieillard-Baron A (2008) Hemodynamic effects of passive leg raising: an echocardiographic study in patients with shock. Intensive Care Med 34:1239–1245
Monnet X, Rienzo M, Osman D, Anguel N, Richard C, Pinsky MR, Teboul JL (2006) Passive leg raising predicts fluid responsiveness in the critically ill. Crit Care Med 34:1402–1407
Lamia B, Ochagavia A, Monnet X, Chemla D, Richard C, Teboul JL (2007) Echocardiographic prediction of volume responsiveness in critically ill patients with spontaneously breathing activity. Intensive Care Med 33:1125–1132
Cecconi M, Rhodes A, Poloniecki J, Della Rocca G, Grounds R (2009) Bench-to-bedside review: The importance of the precision of the reference technique in method comparison studies: with specific reference to the measurement of cardiac output. Crit Care 13:201
Jabot J, Teboul J, Richard C, Monnet X (2009) Passive leg raising for predicting fluid responsiveness: importance of the postural change. Intensive Care Med 35:85–90
Bundgaard-Nielsen M, Holte K, Secher N, Kehlet H (2007) Monitoring of peri-operative fluid administration by individualized goal-directed therapy. Acta Anaesthesiol Scand 51:331–340
Marqué S, Cariou A, Chiche J, Squara P (2009) Non invasive cardiac output monitoring (NICOM) compared to minimally invasive monitoring (VIGILEO). Crit Care 13:R73
Raval N, Squara P, Cleman M, Yalamanchili K, Winklmaier M, Burkhoff D (2008) Multicenter evaluation of noninvasive cardiac output measurement by bioreactance technique. J Clin Monit Comput 10:113–119
Squara P, Denjean D, Estagnasie P, Brusset A, Dib JC, Dubois C (2007) Noninvasive cardiac output monitoring (NICOM): a clinical validation. Intensive Care Med 33:1191–1194
Squara P, Rotcajg D, Denjean D, Estagnasie P, Brusset A (2009) Comparison of monitoring performance of Bioreactance vs. pulse contour during lung recruitment maneuvers. Crit Care 13:125
Squara P, Fourquet E, Jacquet L, Broccard A, Uhlig T, Rhodes A, Bakker J, Perret C (2003) A computer program for interpreting pulmonary artery catheterization data: results of the European HEMODYN Resident Study. Intensive Care Med 29:735–741
Squara P (2008) Bioreactance, a new method for non invasive cardiac output monitoring. In: Vincent J (ed) Year book of intensive care and emergency medicine. Springer, Paris, pp 619–630
Parsonnet V, Bernstein A, Gera M (1996) Clinical usefulness of risk-stratified outcome analysis in cardiac surgery in New Jersey. Ann Thorac Surg 61:S8–11
Nilsson J, Algotsson L, Höglund P, Lührs C, Brandt J (2004) EuroSCORE predicts intensive care unit stay and costs of open heart surgery. Ann Thorac Surg 78:1528–1534
Trof R, Sukul S, Twisk J, Girbes A, Groeneveld A (2010) Greater cardiac response of colloid than saline fluid loading in septic and non-septic critically ill patients with clinical hypovolaemia. Intensive Care Med 36:697–701
Squara P, Cecconi C, Singer M, Rhodes A, Chiche J (2009) Tracking changes in cardiac output; methodological considerations for the validation of monitoring devices. Intensive Care Med 35:1801–1808
Baldi P, Brunak S, Chauvin Y, Andersen C, Nielsen H (2000) Assessing the accuracy of prediction algorithms for classification: an overview. Bioinformatics 16:412–424
Della Rocca G, Costa M (2004) Hemodynamic-volumetric monitoring. Minerva Anesthesiol 70:229–232
McGee W (2009) A simple physiologic algorithm for managing hemodynamics using stroke volume and stroke volume variation: physiologic optimization program. J Intensive Care Med 24:352–360
Muller L, Louart G, Bousquet P, Candela D, Zoric L, de La Coussaye J, Jaber S, Lefrant J (2010) The influence of the airway driving pressure on pulsed pressure variation as a predictor of fluid responsiveness. Intensive Care Med 36:496–503
Greene A, Shoukas A (1986) Changes in canine cardiac function and venous return curves by the carotid baroreflex. Am J Physiol 25:1288–1296
Takata M, Wise R, Robotham J (1990) Effects of abdominal pressure on venous return: abdominal vascular zone conditions. J Appl Physiol 69:1961–1972
Kitano Y, Takata M, Sasaki N, Zhang Q, Yamamoto S, Miyasaka K (1999) Influence of increased abdominal pressure on steady-state cardiac performance. J Appl Physiol 86:1651–1656
Fessler H, Brower R, Shapiro E, Permutt S (1993) Effects of positive end-expiratory pressure and body position on pressure in the thoracic great veins. Am Rev Respir Dis 148:1657–1664
Cecconi M, Dawson D, Grounds R, Rhodes A (2009) Lithium dilution cardiac output measurement in the critically ill patient: determination of precision of the technique. Intensive Care Med 35:498–504
Lakhal K, Ehrmann S, Runge I, Benzekri-Lefevre D, Legras A, Dequin P, Mercie rE, Wolff M, Regnier B, Boulain T (2010) Central venous pressure measurements improve the accuracy of leg raising-induced change in pulse pressure to predict fluid responsiveness. Intensive Care Med 36:940–948
Acknowledgments
The authors thank Steve Novak for his help in collecting data. Cheetah-Medical gave for free the NICOM devices and the consumable.
Conflict of interest
Pierre Squara is a consultant for Cheetah-Medical. Other authors have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Benomar, B., Ouattara, A., Estagnasie, P. et al. Fluid responsiveness predicted by noninvasive Bioreactance-based passive leg raise test. Intensive Care Med 36, 1875–1881 (2010). https://doi.org/10.1007/s00134-010-1990-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-010-1990-6