Abstract
Objective
To test the tolerability and safety of the universal plasma Uniplas [solvent/detergent (SD)-treated plasma], infused regardless of the patient's blood group.
Design
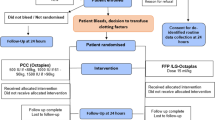
Prospective, parallel group, controlled and observer-blinded study, randomized with respect to patients requiring plasma transfusion.
Setting
Cardiothoracic operating room and ICU in a university hospital.
Patients
Eighty-four patients undergoing open-heart surgery comparing three parallel treatment groups and one control group.
Interventions
The Uniplas treatment group was subdivided into patients with blood group A, B or AB, and group O. The treatment group receiving Octaplas of type AB, was not subdivided. Patients who did not require any plasma transfusion served as control.
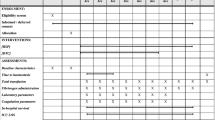
Measurements
Complement activation (C3bc, TCC), direct antiglobulin test (DAT) and other immunohaematological tests, tests for haemolysis, and relevant clinical observations during treatment phase. Blood samples were collected again after 6 months for evaluation of viral safety.
Results
Of the 84 patients, 29 served as control group. Uniplas was transfused in 36 of the patients (1–23 units). Octaplas was transfused in 19 patients (1–11 units). During the study no clinical adverse events related to plasma transfusion were observed. The degree of complement activation C3bc and TCC, a recommended test for biocompatibility, did not show any increased activation after Uniplas or Octaplas transfusion. No haemolytic reactions, positive DAT-tests or viral transmissions were observed after Uniplas transfusion.
Conclusion
In open-heart surgery, Uniplas, which can be transfused regardless of a patient's blood group, was well-tolerated and gave no adverse drug reactions.


Similar content being viewed by others
References
Solheim BG, Svennevig JL, Mohr B, Dragsund M, Noddeland H, Tølløfsrud S (1993) The use of Octaplas in patients undergoing open-heart surgery. In: Muller-Berghaus G, et al (eds) DIC: Pathogenesis, diagnosis and therapy of disseminated intravascular fibrin formation. Elsevier Science, pp 253–262
Horowitz B, Bonoma R, Prince AM, Chin SN, Brotman B, Schulman RW (1992) Solvent/detergent treated plasma: a virus-inactivated substitue for fresh frozen plasma. Blood 79:826–831
Hellstern P, Sachse H, Schwinn H, Oberfrank K (1992) Manufacture and in vitro characterization of solvent/detergent-treated human plasma. Vox Sang 63:178–185
Beeck H, Hellstern P (1998) In vitro characterization of solvent/detergent-treated human plasma and of quarantine fresh frozen plasma. Vox Sang 74[Suppl 1]:219–223
Flesland O, Solheim BG, Seghatchian J (2001) Transfusion medicine in Norway. Time for change. Transf Apher Sci 25:211–214
Heal JM, Blumberg N (1999) The second century of ABO: and now for something completely different. Editorial. Transfusion 39:1155–1159
Baele PL, De Bruyere M, Deneys V, Dupont E, Flament J, Lambermont M, Lantine D, Steensens L, van Camp B, Waterloos H (1994) Bedside transfusion errors. A prospective survey by the Belgium Sanguis group. Vox Sang 66:117–121
Linden JV, Wagner K, Voytovich AE, Sheehan J (2000) Transfusion errors in New Yourk State: an analysis of 10 years' experience. Transfusion 40:1207–1213
HMSO (1973) Dried human plasma. British Pharmacopoeia 67. HMSO, London
Flesland O, Seghatchian J, Solheim BG (2002) The Norwegian plasma fraction project-a 12 year clinical and economic success story. Transf Apher Sci (in press)
Noddeland H, Tølløfsrud S, Svennevig JL, Bentsen G, Brosstad F, Solheim BG (2002) Universal solvent/detergent-treated fresh frozen plasma (Uniplas) – rationale and clinical properties. Thrombosis research.107:S33-S37
Svennevig J, Bech J, Karlsen H, Amlie E, Olsen A (1995) From a registry to a clinical information system. Development of the Datacor system at the surgery department A, Rikshospitalet. Tidsskr Nor Laegefor 115:1057–1059
Garred P, Mollnes TE, Lea T (1988) Quantification in enzyme-linked immunosorbent assay of a C3 neoepitope expressed on activated human complement factor C3. Scand J Immunol 27:329–335
Mollnes TE, Lea T, Frøland SS, Harboe M (1985) Quantification of the terminal complement complex in human plasma by an enzyme-linked immunosorbent assay based on monoclonal antibodies against a neoantigen of the complex. Scand J Immunol 22:197–202
O'Donnell J, Laffan MA (2001) The relationship between ABO histo-blood group, factor VIII, and von Willebrand factor. Transf Med 11:343–351
Breilatt J, Dorson WJ (1984) Quantification of serum complement activation by polymeric membranes and materials. J Am Soc Artif Int Organs 7:57–63
Deppisch R, Scmitt V, Bommer J (1990) Fluid phase generation of terminal complement complex as a novel index of biocompatibility. Kidney Int 37:396–706
Svennevig JL, Tølløfsrud S, Kongsgaard U, Noddeland H, Mohr B (1996) Complement activation during and after open-heart surgery is only marginally affected by the choice of fluid for volume replacement. Perfusion 11:326–332
Gardinali M, Cicardi M, Agostoni A, Hugli TE (1986) Complement activation in extracorporeal circulation: Physiological and pathological implications. Pathol Immunopathol Res 5:352–370
Gasparone A, Ciavolella M, Mercogliano D (1986) Complement activation by different cardiopulmonary priming solutions. Perfusion 1:255–260
Schøtt U, Bersens O, Jaremo P (1987) Blood substitution and complement activation. Acta Anaesthesiol Scand 31:559–566
Horowitz B, Lazo A, Grossberg H, Page G, Lippin A, Swan G (1998) Virus inactivation by solvent/detergent treatment and the manufacture of SD-plasma. Vox Sang 74[Suppl 1]:203–206
Solheim BG, Rollag H, Svennevig JL, Arafa O, Fosse E, Bergerud U (2000) Viral safety of solvent/detergent treated plasma. Transfusion 40:84–90
Acknowledgments
The Authors thank Lise Bjørnskau for excellent laboratory assistance and Dipl. Stat. Rolf Hövelmann, ClinResearch, Cologne, Germany, for statistical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tølløfsrud, S., Noddeland, H., Svennevig, J.L. et al. Universal fresh frozen plasma (Uniplas): a safe product in open-heart surgery. Intensive Care Med 29, 1736–1743 (2003). https://doi.org/10.1007/s00134-003-1952-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-003-1952-3