Abstract
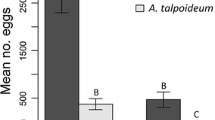
In species with widespread distribution, populations found in markedly different environments can show differences in developmental traits. This, in time, can have an effect on reproductive success. Sources of variation in developmental traits can be genetic or environmentally induced. I examined the relationship between environmental and genetic influences on juvenile development in populations of the colonial spider, Parawixia bistriata, located at sites with different moisture regimes and associated environmental variables (e.g., prey availability). It was expected that individuals from different populations would show differences in developmental traits and that those differences will be associated with lower reproductive success at dry sites. I recorded the phenology and developmental traits of native and transplanted individuals in the field and estimated reproductive success based on clutch size. Colonies from wet versus dry sites showed different phenologies, with individuals at dry sites maturing later. Transplant results suggest plasticity in instar duration caused by environmental effects. Despite differences in resources and spider phenology, clutch sizes of native dry and wet populations were similar. Transplanted individuals, however, were differentially affected. Transplants from wet to dry sites (WD) showed lower growth rates and smaller clutches, whereas transplants from dry to wet sites had larger clutch sizes than in native habitat. Delayed maturation and failure to reproduce in WD individuals is associated with a lower tendency to capture prey in groups and less aggressive interactions during prey capture. Thus, despite negative environmental effects on development, dry native individuals have evolved non-developmental traits that allow successful reproduction.


Similar content being viewed by others
References
Arnett AE, Gotelli NJ (1999) Geographic variation in life-history traits of the ant lion Myrmeleon immaculatus: evolutionary implications of Bergmann's rule. Evolution 53:1180–1188
Arnqvist G, Henriksson S (1997) Sexual cannibalism in the fishing spider and a model for the evolution for sexual cannibalism based on genetic constraints. Evol Ecol 11:255–273
Berner D, Blanckenhorn WU (2007) An ontogenetic perspective on the relationship between age and size at maturity. Funct Ecol 21:505–512
Berven KA (1982) The genetic basis of altitudinal variation in the wood frog Rana sylvatica I. Experimental analysis of life history traits. Evolution 36:962–983
Cabrera AL (1971) Fitogeografía de la República Argentina. Bol Soc Argent Bot 14:1–42
Carroll SP, Corneli PS (1999) The evolution of behavioral norms of reaction as a problem in ecological genetics: theory, methods and data. In: Foster SA, Endler JA (eds) Geographic variation in behavior: perspectives on evolutionary mechanisms. Oxford University Press, New York, pp 52–68
Castillo JA, Eberhard WG (1983) Use of artificial webs to determine prey available to orb weaving spiders. Ecology 64:1655–1658
Conover WJ, Iman RL (1981) Rank transformations as a bridge between parametric and nonparametric statistics. Am Stat 35:124–129
Crozier LG (2004) Field transplants reveal summer constraints on a butterfly range expansion. Oecologia 141:148–157
Davidowitz G, Nijhout HF (2004) The physiological basis of reaction norms: the interaction among growth rate, the duration of growth and body size. Integrative and Comparative Biology 44:443–449
Debat V, David P (2001) Mapping phenotypes: canalization, plasticity and developmental stability. Trends Ecol Evol 16:555–561
Fernández Campón F (2005) Variation in life history and behavioral traits in the colonial spider Parawixia bistriata (Araneidae): some adapting responses to different environments. Ph.D. thesis, University of Tennessee, Knoxville
Fernández Campón F (2007) Group foraging in the colonial spider Parawixia bistriata (Araneidae): effect of resource levels and prey size. Anim Behav 74:1551–1562
Fernández Campón F (2008) More sharing when there is less: insights on spider sociality from an orb-weaver's perspective. Anim Behav 75:1063–1073
Fernández-Montraveta C, Moya-Laraño J (2007) Sex-specific plasticity of growth and maturation size in a spider: implications for sexual size dimorphism. J Evol Biol 20:1689–1699
Fordyce JA (2003) Aggregative feeding of pipevine swallowtail larvae enhances hostplant suitability. Oecologia 135:250–257
Fowler HG, Gobbi N (1988) Communication and synchronized molting in a colonial araneid spider, Eriophora bistriata. Experientia 44:720–722
Hassall M, Helden A, Benton T (2003) Phenotypic plasticity and interpopulation differences in life history traits of Armadillidium vulgare (Isopoda: Oniscidae). Oecologia 137:85–89
Hassall M, Helden AJ, Goldson A, Grant A (2005) Ecotypic differentiation and phenotypic plasticity in reproductive traits of Armadillilium vulgare (Isopoda: Oniscidea). Oecologia 142:51–60
Hedrick AV, Riechert SE (1989) Genetically based variation between two spider populations in foraging behavior. Oecologia 80:533–539
Henschel JR, Lubin YD, Schneider J (1995) Sexual competition in an inbreeding social spider, Stegodyphus dumicola (Araneae, Eresidae). Insect Soc 42:419–426
Higgins LE (1992) Developmental plasticity and fecundity in the orb-weaving spider Nephila clavipes. J Arachnol 20:94–106
Higgins LE (1993) Constraints and plasticity in the development of juvenile Nephila clavipes in Mexico. J Arachnol 21:107–119
Higgins LE (2000) The interaction of season length and development time alters size at maturity. Oecologia 122:51–59
Higgins LE, Rankin MA (1996) Different pathways in arthropod postembryonic development. Evolution 50:573–582
Levi HW (1992) Spiders of the orb-weaver genus Parawixia in America (Araneae: Araneidae). Bull Mus Comp Zool 153:1–46
Mayntz D, Toft S, Vollrath F (2003) Effects of prey quality and availability on the life history of a trap-building predator. Oikos 101:631–638
Miyashita T (1968) Growth and development of Lycosa T-insignita BOES. et STR. (Araneae: Lycosidae) under different feeding conditions. Appl Entomol Zool 3:81–88
Moya-Laraño J, Macías-Ordóñez R, Blanckenhorn WU, Fernández-Montraveta C (2008) Analysing body condition: mass, volume or density? J Anim Ecol 77:1099–1108
Pardo LM, Johnson LE (2005) Explaining variation in life-history traits: growth rate, size, and fecundity in a marine snail across an environmental gradient lackin predators. Mar Ecol Prog Ser 296:229–239
Rice WR (1989) Analysing tables of statistical tests. Evolution 43:223–225
Riechert SE, Hall RF (2000) Local population success in heterogeneous habitats: reciprocal transplant experiments completed on a desert spider. J Evol Biol 13:541–550
Riechert SE, Tracy CR (1975) Thermal balance and prey availability: bases for a model relating web-site characteristics to spider reproductive success. Ecology 56:265–284
Sandoval CP (1987) Aspectos da ecologia e socialidade de uma aranha colonial: Eriophora bistriata (Rengger, 1936). Master Sc., Universidade Estadual de Campinas
Stearns SC, Koella JC (1986) The evolution of phenotypic plasticity in life-history traits: predictions of reaction norms for age and size at maturity. Evolution 40:893–913
Stokes ME, Davis CS, Koch GG (2000) Categorical data analysis using the SAS system. SAS Institute Inc, Cary
Uetz WG, Papke R, Kilinc B (2002) Influence of feeding regime on body size, body condition and a male secondary sexual character in Schizocosa ocreata wolf spiders (Araneae, Lycosidae): condition-dependence in a visual signaling trait. J Arachnol 30:461–469
Uhl G, Schmitt S, Schafer MA, Blanckenhorn W (2004) Food and sex-specific growth strategies in a spider. Evol Ecol Res 6:523–540
Acknowledgements
The original version of this manuscript was greatly improved by comments and suggestions from Susan Riechert. I would also like to thank Nadia Ayoub, Linden Higgins, and three anonymous referees for their comments. I am grateful to Patricia Lange, Federico Paredes, Alexa Ravelo, and Mirna Maribel Riquelme for their help and enthusiasm while working in the field. Thanks to the Allende and DiGiácomo families, Aves Argentinas, and Dirección de Fauna of the Formosa province for allowing me permission to work in the different sites and for their logistic support. Funding was provided by the Department of Ecology and Evolutionary Biology of the University of Tennessee through a summer grant and a doctoral fellowship by CONICET from Argentina. The experiments carried out during the study comply with the current laws of Argentina.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
S1
Climatograms for dry (a) and wet (b) sites during the period 1988–2000. Bars correspond to average monthly precipitation, and the line represents average daily temperature per month. Wet site 2 data source: DiGiácomo 2001; dry sites data source: J. Pérez, unpublished data; collected in a site located 2 km north of the site Dry 1. (DOC 103 kb)
S2
Temperature and precipitation between 1988 and 2000 for wet site 2 and a site adjacent to the dry sites. (DOC 31 kb)
S3
Repeated measures analysis of variance of insect dry biomass sampled per trap per night. (DOC 32 kb)
S4
Insect dry biomass (gram) sampled per trap per night in dry and wet sites. (DOC 30 kb)
S5
Contrasts of parameter estimates for developmental stage relative to the interaction effect rearing environment × origin for native and transplanted colonies of both habitats. (DOC 32 kb)
S6
Change in spider mass of (black symbols) and transplanted (open symbols) individuals from dry (a) and wet (b) habitat of origin as a function of time (days since beginning of the study season staring on Oct 15th). Solid lines are the estimated regression functions and 95% confidence intervals for native colonies; dash lines are regression functions and 95% confidence intervals for transplanted colonies. (DOC 287 kb)
S7
Cephalothorax width by instar of individuals originally from dry (a) and wet habitat (b). Number above bars indicates sample sizes; error bars indicates standard errors. (DOC 51 kb)
S8
Frequency of parasitism in egg sacs produced by native and transplanted individuals in dry and wet sites. (DOC 33 kb)
S9
Generalized linear mixed model analysis of clutch size of parasitized and non-parasitized sacs. (DOC 32 kb)
S10
Size of native colonies found in the dry and wet sites. (DOC 30 kb)
Rights and permissions
About this article
Cite this article
Fernández Campón, F. Cross-habitat variation in the phenology of a colonial spider: insights from a reciprocal transplant study. Naturwissenschaften 97, 279–289 (2010). https://doi.org/10.1007/s00114-009-0640-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00114-009-0640-8