Abstract
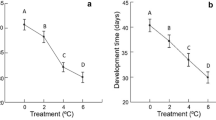
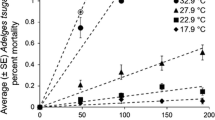
The geographic ranges of most species are expected to shift to higher elevations and latitudes in response to global warming. But species react to specific environmental changes in individualistic ways, and we are far from a detailed understanding of range-shifts. Summer temperature often limits the ranges of insects and plants, so many range-shifts are expected to track summer warming. I explore this potential range-limiting factor in a case study of a northwardly expanding American butterfly, Atalopedes campestris (Lepidoptera, Hesperiidae). This species has recently colonized the Pacific Northwest, USA, where the mean annual temperature has risen 0.8–1.8°C over the past 100 years. Using field transplant experiments across the current range edge, I measured development time, survivorship, fecundity and predation rates along a naturally occurring thermal gradient of 3°C. Development time was significantly slower outside the current range in eastern Washington (WA), as expected because of cooler temperatures there. Slower development would reduce the number of generations possible per year outside the current range, dramatically lowering the probability that a population could survive there. Differences in survivorship, fecundity and predation rate across the range edge were not significant. The interaction between summer and winter temperature appears to be crucial in defining the current range limit. The estimated difference in temperature required to affect the number of generations is greater than the extent of summer warming observed over the past century, however, and thus historically winter temperature alone probably limited the range in southeastern WA. Nonetheless, extraordinarily warm summers may have improved colonization success, increasing the probability of a range expansion. These results suggest that extreme climatic events may influence rates of response to long-term climate change. They also demonstrate that range-limiting factors can change over time, and that the asymmetry in seasonal warming trends will have biological consequences.





Similar content being viewed by others
References
Andrewartha HG, Birch LC (1954) The distribution and abundance of animals. University of Chicago Press, Chicago, Ill.
Atkinson D (1994) Temperature and organism size—a biological law for ectotherms? Adv Ecol Res 25:1–58
Baker BC (ed) (1999) Area-averaged temperature time series for China, India, and the United States. National Climatic Data Center, National Oceanic and Atmospheric Administration, Asheville
Bateman MA (1967) Adaptations to temperature in geographic races of the Queensland fruit fly, Dacus (Strumeta) tyroni. Aust J Zool 15:1141–1161
Beaumont LJ, Hughes L (2002) Potential changes in the distributions of latitudinally restricted Australian butterfly species in response to climate change. Global Change Biol 8:954–971
Berrigan D, Charnov EL (1994) Reaction norms for age and size at maturity in response to temperature: a puzzle for life historians. Oikos 70:474–478
Berry PM, Dawson TP, Harrison PA, Pearson RG (2002) Modelling potential impacts of climate change on the bioclimatic envelope of species in Britain and Ireland. Global Ecol Biogeogr 11:453–462
Botkin D, Janak JF, Wallis JR (1972) Rationale, limitations and assumptions of a northeastern forest growth simulator. IBM J Res Dev 16:101–116
Cammell ME, Knight JD (1992) Effects of climatic change on the population dynamics of crop pests. Adv Ecol Res 22:117–162
Chown SL, Addo-Bediako A, Gaston KJ (2002) Physiological variation in insects: large-scale patterns and their implications. Comp Biochem Physiol B Biochem Mol Biol 131:587–602
Crozier L (2001) Climate change and species’ range boundaries: a case study at the northern range limits of Atalopedes campestris (Lepidoptera: Hesperiidae), the sachem skipper. PhD thesis. University of Washington, Wash.
Crozier L (2003) Winter warming facilitates range expansion: cold tolerance of the butterfly Atalopedes campestris. Oecologia 135:648–656
Crozier L (2004) Warmer winters drive butterfly range expansion by increasing survivorship. Ecology 85:231–241
Davis MB (1986) Climatic instability, time lags, and community disequilibrium. In: Diamond J, Case TJ (eds) Community ecology. Harper and Row, New York, pp 269–284
Davis AJ, Lawton JH, Shorrocks B, Jenkinson LS (1998) Individualistic species responses invalidate simple physiological models of community dynamics under global environmental change. J Anim Ecol 67:600–612
Denno RF, Douglass LW, Jacobs D (1985) Crowding and host plant nutrition: environmental determinants of wing form in Prokelisia marginata. Ecology 66:1588–1596
Dornfeld EJ (1980) Butterflies of Oregon. Timber Press, Forest Grove
Ehrlich PR (1980) Extinction, reduction, stability and increase: the responses of checkerspot butterfly (Euphydryas) populations to the California drought. Oecologia 46:101–105
Fischer K, Fiedler K (2002) Reaction norms for age and size at maturity in response to temperature: a test of the compound interest hypothesis. Evol Ecol 16:333–349
Forister ML, Shapiro AM (2003) Climatic trends and advancing spring flight of butterflies in lowland California. Global Change Biol 9:1130–1135
Franklin JF, Swanson FJ, Harmon ME, Petty DA, Spies TA, Dale VH, McKee A, Ferrell WK, Means JE, Gregory SV, Lattin JD, Schowalter TD, Larsen D (1992) Effects of global climatic change on forests in northwestern North America. In: Peters RL, Lovejoy TE (eds) Global warming and biological diversity. Yale University Press, New Haven, Conn., pp 244–257
Garcia-Barros E (2000) Body size, egg size, and their interspecific relationships with ecological and life history traits in butterflies (Lepidoptera: Papilionoidea, Hesperioidea). Biol J Linn Soc 70:251–284
Gilbert N (1980) Comparative dynamics of a single-host aphid. I. The evidence. J Anim Ecol 49:351–369
Groisman PY, Karl TR, Easterling DR, Knight RW, Jamason PF, Hennessy KJ, Suppiah R, Page CM, Wibig J, Fortuniak K, Razuvaev VN, Douglas A, Forland E, Zhai PM (1999) Changes in the probability of heavy precipitation: important indicators of climatic change. Climatic Change 42:243–283
Hagstrum DW, Milliken GA (1991) Modeling differences in insect developmental times between constant and fluctuating temperatures. Ann Entomol Soc Am 84:369–379
Hanford Meteorological Station (1998) Hourly subsurface soil temperature at 0.5 inch depth. Pacific Northwest National Laboratory, Richland, Wash.
Hinchliff J (1994) An atlas of Oregon butterflies. Evergreen Aurelians, Corvallis, Ore.
Hinchliff J (1996) An atlas of Washington butterflies. Evergreen Aurelians, Corvallis, Ore.
Hoffmann AA, Blows MW (1994) Species borders: ecological and evolutionary perspectives. Trees 9:223–227
Honk A (1996) Geographical variation in thermal requirements for insect development. Eur J Entomol 93:303–312
Igbinosa IB (1986) On the relationship between fecundity and size of the nettle moth Latoia viridissima Lepidoptera: Limacodidae. Rev Zool Afr 99:359–364
IPCC (2001a) Climate change 2001: impacts, adaptation and vulnerability. Cambridge University Press, Cambridge
IPCC (2001b) Climate change 2001: the scientific basis. Cambridge University Press, Cambridge
Karl TR, Jones PD, Knight RW, Kukla G, Plummer M, Razuvayev V, Gallo KP, Lindseay J, Charlson RJ, Peterson TC (1993) Asymmetric trends of daily maximum and minimum temperature. Bull Am Meteorol Soc 74:1007–1023
Kingsolver JG (1983a) Ecological significance of flight activity in Colias butterflies: implications for reproductive strategy and population structure. Ecology 64:546–551
Kingsolver JG (1983b) Thermoregulation and flight in Colias butterflies: elevational patterns and mechanistic limitations. Ecology 64:534–545
Lawton JH (1995) The response of insects to environmental change. In: Harrington R, Stork NE (eds) Insects in a changing environment. Academic Press, Harcourt Brace, New York, pp 3–26
Logan J, Powell J (2001) Ghost forests, global warming, and the mountain pine beetle. Am Entomol 47:160–173
McKown S (1992) 1991 season summary. News Lepid Soc 2
McLaughlin JF, Hellmann JJ, Boggs CL, Ehrlich PR (2002) Climate change hastens population extinctions. PNAS 99:6070–6074
Meats A (1989) Abiotic mortality factors—temperature. In: Robinson AS, Hooper G (eds) Fruit flies; their biology, natural enemies, and control. Elsevier, Amsterdam, pp 229–240
Menzel A, Fabian P (1999) Growing season extended in Europe. Nature 397:659
Mousseau TA (1997) Ectotherms follow the converse to Bergmann’s rule. Evolution 51:630–632
NCDC (2002) Global historical climatological network data. National Oceanic and Atmospheric Administration, National Climatic Data Center http://www.ncdc.noaa.gov
Nylin S, Svard L (1991) Latitudinal patterns in the size of European butterflies. Holarct Ecol 14:192–202
Parmesan C (1996) Climate and species’ range. Nature 382:765–766
Parmesan C, Yohe G (2003) A globally coherent fingerprint of climate change impacts across natural systems. Nature 421:37–42
Parmesan C, Ryrholm N, Stefanescu C, Hill JK, Thomas CD, Descimon H, Huntley B, Kaila L, Kullberg J, Tammaru T, Tennent WJ, Thomas JA, Warren M (1999) Poleward shifts in geographical ranges of butterfly species associated with regional warming. Nature 399:579–583
Parmesan C, Root T, Willig MR (2000) Impacts of extreme weather and climate on terrestrial biota. Bull Am Meteorol Soc 81:443–450
Pollard E (1979) Population ecology and change in range of the white admiral butterfly Ladoga camilla L. in England. Ecol Entomol 4:61–74
Pollard E (1988) Temperature, rainfall, and butterfly numbers. J Appl Ecol 25:819–828
Pollard E, Yates T (1993) Monitoring butterflies for ecology and conservation. Chapman and Hall, London
Porter J (1995) The effects of climate change on the agricultural environment for crop insect pests with particular reference to the European corn borer and grain maize. In: Harrington R, Stork N (eds) Insects in a changing environment. Academic Press, New York, pp 93–123
Rogers DJ, Randolph SE (1986) Distribution and abundance of tsetse flies. J Anim Ecol 55:1007–1025
Root T, Price JT, Hall KR, Schneider S, Rosenzweig C, Pounds JA (2003) Fingerprints of global warming on wild animals and plants. Nature 421:57–60
Roy DB, Sparks T (2000) Phenology of British butterflies and climate change. Global Change Biol 6:407–416
Scott JA (1979) Hibernal diapause of North American Papilionoidea and Hesperioidea. J Res Lepidoptera 18:171–200
Scott JA (1986) The butterflies of North America: a natural history and field guide. Stanford University Press, Stanford, Mass.
Singer MC, Thomas CD (1996) Evolutionary responses of a butterfly metapopulation to human- and climate-caused environmental variation. Am Nat 148:9–39
Taylor P (1993) The Sachem: a new skipper for Manitoba and the prairie provinces. Blue Jay 51:193–195
Thomas CD, Singer MC, Boughton DA (1996) Catastrophic extinction of population sources in a butterfly metapopulation. Am Nat 148:957–975
Worner SP (1992) Performance of phenological models under variable temperature regimes: consequences of the Kaufmann or rate summation effect. Environ Entomol 21:689–699
WRCC (2001) Daily temperature and precipitation data. Western Regional Climate Center. http://www.wrcc.sage.dri.edu
Acknowledgements
I am grateful to Joel Kingsolver and Jon Hoekstra for providing invaluable suggestions and support throughout the project. T. Unruh, C. Duberstein, D. Honaker, M. Tari, J. Zach, S. Smith, D. Johns, C. Hilgert and A. Savos helped monitor experiments. I thank many landowners for allowing me to survey and conduct experiments on their property, especially Pacific Northwest National Laboratories and the Lutheran Church of the Master in Richland, USDA-ARS in Yakima, the Yakima Arboretum, Central Washington University in Ellensburg, Dot Honaker and Jim Dillman. Michael Dillon and two anonymous reviewers greatly improved this manuscript. An NSF predoctoral fellowship, the National Wildlife Foundation, the Xerces Society, and a USDA NRICGP grant funded this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Crozier, L.G. Field transplants reveal summer constraints on a butterfly range expansion. Oecologia 141, 148–157 (2004). https://doi.org/10.1007/s00442-004-1634-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-004-1634-z