Abstract
Introduction
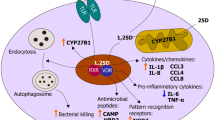
The steroid hormone metabolite of vitamin D3, 1α,25-dihydroxyvitamin D3 (1,25D3), promotes osteogenic activity and regulates calcium and phosphate metabolism, which are actions regarded as classical vitamin D-regulated functions. Besides its role in these processes, 1,25D3 also seems implicated in the host defense against microbial/pro-inflammatory attacks. Low serum levels of vitamin D3 (vitamin D deficiency) are associated with osteoporosis and increased risk of fractures but also inflammatory diseases and their disease progression, presumably via mechanisms associated with 1,25D3-evoked modulation of the innate immune system. 1,25D3 has been reported to modulate many inflammatory responses, suggesting that it regulates multiple transcriptional targets within the inflammatory system.
Results
Experimental studies in various experimental systems show that 1,25D3 differentially regulates the production of pro-inflammatory cytokines and chemokines depending on cell type. Importantly, many reports show that 1,25D3 up-regulates expression of the human antimicrobial peptide hCAP-18/LL-37 gene. The hCAP-18/LL-37 gene seems indeed to be an important transcriptional target for 1,25D3. However, only limited evidence is presented showing that 1,25D3 consistently increases the amount of biologically active LL-37 peptide.
Conclusion
In the present review, we discuss 1,25D3-induced down-regulation of cytokine/chemokine production and stimulation of hCAP-18/LL-37 gene expression which represent two very important pathways for 1,25D3-evoked regulation of the innate immune response.


Similar content being viewed by others
References
Carlberg C, Campbell MJ. Vitamin D receptor signaling mechanisms: Integrated actions of a well-defined transcription factor. Steroids. 2013;78:127–36.
Gurlek A, Pittelkow MR, Kumar R. Modulation of growth factor/cytokine synthesis and signaling by 1alpha,25-dihydroxyvitamin D(3): implications in cell growth and differentiation. Endocr Rev. 2002;23:763–86.
Baeke F, Takiishi T, Korf H, Gysemans C, Mathieu C. Vitamin D: modulator of the immune system. Curr Opin Pharmacol. 2010;10:482–96.
Wöbke TK, Sorg BL, Steinhilber D. Vitamin D in inflammatory diseases. Front Physiol. 2014;5:244.
White JH. Vitamin D as an inducer of cathelicidin antimicrobial peptide expression: past, present and future. J Steroid Biochem Mol Biol. 2010;121:234–8.
Van der Does AM, Bergman P, Agerberth B, Lindbom L. Induction of the human cathelicidin LL-37 as a novel treatment against bacterial infections. J Leukoc Biol. 2012;92:735–42.
Adams JS, Sharma OP, Gacad MA, Singer FR. Metabolism of 25-hydroxyvitamin D3 by cultured pulmonary alveolar macrophages in sarcoidosis. J Clin Invest. 1983;72:1856–60.
Zehnder D, Bland R, Williams MC, McNinch RW, Howie AJ, Stewart PM, et al. Extrarenal expression of 25-hydroxyvitamin d(3)-1 alpha-hydroxylase. J Clin Endocrinol Metab. 2001;86:888–94.
Liu PT, Stenger S, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–3.
Bouillon R, Van Assche FA, Van Baelen H, Heyns W, De Moor P. Influence of the vitamin D-binding protein on the serum concentration of 1,25-dihydroxyvitamin D3. J Clin Invest. 1981;67:589–96.
Bikle DD, Gee E, Halloran B, Kowalski MA, Ryzen E, Haddad JG. Assessment of the free fraction of 25-hydroxyvitamin D in serum and its regulation by albumin and the vitamin D-binding protein. J Clin Endocrinol Metab. 1986;63:954–9.
Schmidt-Gayk H, Bouillon R, Roth HJ. Measurement of vitamin D and its metabolites (calcidiol and calcitriol) and their clinical significance. Scand J Clin Lab Invest. 1997;57:33–45.
Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81.
Van Hoof HJ, Van der Mooren MJ, Swinkels LM, Rolland R, Benraad TJ. Hormone replacement therapy increases serum 1,25-dihydroxyvitamin D: A 2-year prospective study. Calcif Tissue Int. 1994;55:417–9.
Chun RF, Peercy BE, Adams JS, Hewison M. Vitamin D binding protein and monocyte response to 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D: analysis by mathematical modeling. PLoS ONE. 2012;7:e30773.
Haussler MR, Kerr Whitfield G, Kaneko I, Haussler CA, Hsieh D, Hsieh J-C, et al. Molecular mechanisms of vitamin D action. Calcif Tissue Int. 2013;92:77–98.
Norman AW. Minireview: vitamin D receptor: new assignments for an already busy receptor. Endocrinology. 2006;147:5542–8.
Doroudi M, Schwartz Z, Boyan BD. Membrane-mediated actions of 1,25-dihydroxy vitamin D3: a review of the roles of phospholipase A2 activating protein and Ca(2 +)/calmodulin-dependent protein kinase II. J Steroid Biochem Mol Biol. 2015;147:81–4.
Dietrich T, Joshipura KJ, Dawson-Hughes B, Bischoff-Ferrari HA. Association between serum concentrations of 25-hydroxyvitamin D3 and periodontal disease in the US population. Am J Clin Nutr. 2004;80:108–13.
Jimenez M, Giovannucci E, Krall Kaye E, Joshipura KJ, Dietrich T. Predicted vitamin D status and incidence of tooth loss and periodontitis. Public Health Nutr. 2014;17:844–52.
Zhan Y, Samietz S, Holtfreter B, Hannemann A, Meisel P, Nauck M, et al. Prospective study of serum 25-hydroxy vitamin D and tooth loss. J Dent Res. 2014;93:639–44.
Jönsson D, Aggarwal P, Nilsson BO, Demmer RT. Beneficial effects of hormone replacement therapy on periodontitis are vitamin D associated. J Periodontol. 2013;84:1048–57.
Antonoglou GN, Suominen AL, Knuuttila M, Ylöstalo P, Ojala M, Männistö S, et al. Associations between serum 25-hydroxyvitamin d and periodontal pocketing and gingival bleeding: results of a study in a non-smoking population in Finland. J Periodontol. 2015;86:755–65.
Xu H, Soruri A, Gieseler RKH, Peters JH. 1,25-dihydroxyvitamin D3 exerts opposing effects to IL-4 on MHC class-II antigen expression, accessory activity, and phagocytosis of human monocytes. Scand J Immunol. 1993;38:535–40.
Griffin MD, Xing N, Kumar R. Vitamin D and its analogs as regulators of immune activation and antigen presentation. Annu Rev Nutr. 2003;23:117–35.
Zhu K, Gläser R, Mrowietz U. Vitamin D(3) and analogues modulate the expression of CSF-1 and its receptor in human dendritic cells. Biochem Biophys Res Commun. 2002;297:1211–7.
Khoo AL, Chai LY, Koenen HJ, Oosting M, Steinmeyer A, Zuegel U, et al. Vitamin D(3) down-regulates proinflammatory cytokine response to Mycobacterium tuberculosis through pattern recognition receptors while inducing protective cathelicidin production. Cytokine. 2011;55:294–300.
Müller K, Bendtzen K. 1,25-dihydroxyvitamin D3 as a natural regulator of human immune functions. J Investig Dermatol Symp Proc. 1996;1:68–71.
Di Rosa M, Malaguarnera G, De Gregorio C, Palumbo M, Nunnari G, Malaguarnera L. Immuno-modulatory effects of vitamin D3 in human monocyte and macrophages. Cell Immunol. 2012;280:36–43.
Zhang Y, Leung DY, Richers BN, Liu Y, Remigio LK, Riches DW, et al. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J Immunol. 2012;188:2127–35.
Zhang Y, Leung DY, Goleva E. Vitamin D enhances glucocorticoid action in human monocytes: involvement of granulocyte-macrophage colony-stimulating factor and mediator complex subunit 14. J Biol Chem. 2013;288:14544–53.
Marcotorchino J, Gouranton E, Romier B, Tourniaire F, Astier J, Malezet C, et al. Vitamin D reduces the inflammatory response and restores glucose uptake in adipocytes. Mol Nutr Food Res. 2012;56:1771–82.
Ding C, Wilding JP, Bing C. 1,25-dihydroxyvitamin D3 protects against macrophage-induced activation of NFκB and MAPK signalling and chemokine release in human adipocytes. PLoS ONE. 2013;8:e61707.
Geilen CC, Bektas M, Wieder T, Kodelja V, Goerdt S, Orfanos CE. 1alpha,25-dihydroxyvitamin D3 induces sphingomyelin hydrolysis in HaCaT cells via tumor necrosis factor alpha. J Biol Chem. 1997;272:8997–9001.
Larsen CG, Kristensen M, Paludan K, Deleuran B, Thomsen MK, Zachariae C, et al. 1,25(OH)2-D3 is a potent regulator of interleukin-1 induced interleukin-8 expression and production. Biochem Biophys Res Commun. 1991;176:1020–6.
Jönsson D, Nebel D, Bratthall G, Nilsson BO. The human periodontal ligament cell: a fibroblast-like cell acting as an immune cell. J Periodontal Res. 2011;46:153–7.
Tang X, Pan Y, Zhao Y. Vitamin D inhibits the expression of interleukin-8 in human periodontal ligament cells stimulated with Porphyromonas gingivalis. Arch Oral Biol. 2013;58:397–407.
Andrukhov O, Andrukhova O, Hulan U, Tang Y, Bantleon HP, Rausch-Fan X. Both 25-hydroxyvitamin-D3 and 1,25-dihydroxyvitamin-D3 reduces inflammatory response in human periodontal ligament cells. PLoS ONE. 2014;9:e90301.
Nebel D, Svensson D, Arosenius K, Larsson E, Jönsson D, Nilsson BO. 1α,25-dihydroxyvitamin D3 promotes osteogenic activity and downregulates proinflammatory cytokine expression in human periodontal ligament cells. J Periodontal Res. 2015;50:666–73.
Zanetti M. The role of cathelicidins in the innate host defenses of mammals. Curr Issues Mol Biol. 2005;7:179–96.
Sorensen OE, Follin P, Johnsen AH, Calafat J, Tjabringa GS, Hiemstra PS, et al. Human cathelicidin, hCAP-18, is processed to the antimicrobial peptide LL-37 by extracellular cleavage with proteinase 3. Blood. 2001;97:3951–9.
Turner J, Cho Y, Dinh NN, Waring AJ, Lehrer RI. Activities of LL-37, a cathelicidin-associated antimicrobial peptide of human neutrophils. Antimicrob Agents Chemother. 1998;42:2206–14.
Nizet V, Ohtake T, Lauth X, Trowbridge J, Rudisill J, Dorschner RA, et al. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414:454–7.
Burton MF, Steel PG. The chemistry and biology of LL-37. Nat Prod Rep. 2009;26:1572–84.
Säll J, Carlsson M, Gidlöf O, Holm A, Humlén J, Öhman J, et al. The antimicrobial peptide LL-37 alters human osteoblast Ca2+ handling and induces Ca2+-independent apoptosis. J Innate Immun. 2013;5:290–300.
Larrick JW, Hirata M, Balint RF, Lee J, Zhong J, Wright SC. Human CAP18: a novel antimicrobial lipopolysaccharide-binding protein. Infect Immun. 1995;63:1291–7.
Nijnik A, Pistolic J, Filewod NCJ, Hancock REW. Signaling pathways mediating chemokine induction in keratinocytes by cathelicidin LL-37 and flagellin. J Innate Immun. 2012;4:377–86.
Tang X, Basavarajappa D, Haegggström JZ, Wan M. P2X7 receptor regulates internalization of antimicrobial peptide LL-37 by human macrophages that promotes intracellular pathogen clearance. J Immunol. 2015;195:1191–201.
Coffelt SB, Tomchuck SL, Zwezdaryk KJ, Danka ES, Scandurro AB. Leucine leucine-37 uses formyl peptide receptor-like 1 to activate signal transduction pathways, stimulate oncogenic gene expression, and enhance the invasiveness of ovarian cancer cells. Mol Cancer Res. 2009;7:907–15.
Jönsson D, Nilsson BO. The antimicrobial peptide LL-37 is anti-inflammatory and proapoptotic in human periodontal ligament cells. J Periodontal Res. 2012;47:330–5.
Inomata M, Into T, Murakami Y. Suppressive effect of the antimicrobial peptide LL-37 on expression of IL-6, IL-8 and CXCL10 induced by Porphyromonas gingivalis cells and extracts in human gingival fibroblasts. Eur J Oral Sci. 2010;118:574–81.
Türkoglu O, Emingil G, Kutukculer N, Atilla G. Gingival crevicular fluid levels of cathelicidin LL-37 and interleukin-18 in patients with chronic periodontitis. J Periodontol. 2009;80:969–76.
Lowry MB, Guo C, Borregaard N, Gombart AF. Regulation of the human cathelicidin antimicrobial peptide gene by 1α, 25-dihydroxyvitamin D3 in primary immune cells. J Steroid Biochem Mol Biol. 2014;143:183–91.
Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005;19:1067–77.
Karlsson J, Carlsson G, Larne O, Andersson M, Pütsep K. Vitamin D3 induces pro-LL-37 expression in myeloid precursors from patients with severe congenital neutropenia. J Leukoc Biol. 2008;84:1279–86.
Lee WJ, Cha HW, Sohn MY, Lee SJ, Kim DW. Vitamin D increases expression of cathelicidin in cultured sebocytes. Arch Dermatol Res. 2012;304:627–32.
Wang TT, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173:2909–12.
Chun RF, Lauridsen AL, Suon L, Zella LA, Pike JW, Modlin RL, et al. Vitamin D-binding protein directs monocyte responses to 25-hydroxy- and 1,25-dihydroxyvitamin D. J Clin Endocrinol Metab. 2010;95:3368–76.
Liu PT, Stenger S, Tang DH, Modlin RL. Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J Immunol. 2007;179:2060–3.
Jeong MS, Kim JY, Lee HI, Seo SJ. Calcitriol may down-regulate mRNA over-expression of toll-like receptor-2 and -4, LL-37 and proinflammatory cytokines in cultured human keratinocytes. Ann Dermatol. 2014;26:296–302.
Bucki R, Namiot DB, Namiot Z, Savage PB, Janmey PA. Salivary mucins inhibit antibacterial activity of the cathelicidin-derived LL-37 peptide but not the cationic steroid CSA-13. J Antimicrob Chemother. 2008;62:329–35.
Yuk JM, Shin DM, Lee HM, Yang CS, Jin HS, Kim KK, et al. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe. 2009;6:231–43.
Acknowledgments
The authors are supported by grants from the Crafoord Foundation, the Swedish Dental Society, the Foundation of Greta and Johan Kock, and the Southern Region within the Swedish Dental Association.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: Andrew Roberts.
Rights and permissions
About this article
Cite this article
Svensson, D., Nebel, D. & Nilsson, BO. Vitamin D3 modulates the innate immune response through regulation of the hCAP-18/LL-37 gene expression and cytokine production. Inflamm. Res. 65, 25–32 (2016). https://doi.org/10.1007/s00011-015-0884-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-015-0884-z