Summary
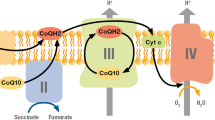
Ubiquinone is a carrier of the mitochondrial respiratory chain which regulates oxidative phosphorylation: it also acts as a membrane stabilizer preventing lipid peroxidation. In man the quinone ring originates from tyrosine, while the formation of the polyisoprenoid lateral chain starts from acetyl CoA and proceeds through mevalonate and isopentenylpyrophosphate; this biosynthetic pathway is the same as the cholesterol one. We therefore performed this study to evaluate whether statins (hypocholesterolemic drugs that inhibit 3-hydroxy-3-methylglutaryl coenzyme A reductase) modify blood levels of ubiquinone. Thirty unrelated outpatients with primary hypercholesterolemia (IIa phenotype) were treated with 20 mg of simvastatin for a 3-month period (group S) or with 20 mg of simvastatin plus 100 mg CoQ10 (group US). The following parameters were evaluated at time 0, and at 45 and 90 days: total plasma cholesterol, high-density lipoprotein-cholesterol, low-density lipoprotein-cholesterol, triglycerides, Apo A1, Apo B and CoQ10 in plasma and in platelets. In the S group, there was a marked decrease in total cholesterol low-density lipoprotein-cholesterol and in plasma CoQ10 levels from 1.08 mg/dl to 0.80 mg/dl. In contrast, in the US group we observed a significant increase of plasma CoQ10 (from 1.20 to 1.48 mg/dl) while the hypocholesterolemic effect was similar to that observed in the S group. Platelet CoQ10 also decreased in the S group (from 104 to 90 ng/mg) and increased in the US group (from 95 to 145 ng/mg). This study demonstrated that simvastatin lowered both low-density lipoprotein-cholesterol and Apo B plasma levels and plasma and platelet levels of CoQ10, and that CoQ10 prevent both plasma and platelet CoQ10 reduction, without affecting the hypocholesterolemic effect of simvastatin.
Similar content being viewed by others
References
Lipid Research Clinics Coronary Prevention Trial. I. Reduction in incidence of coronary heart disease. II. The relationship of reduction in incidence of coronary heart disease to cholesterol lowering. JAMA 1984;251:351.
Muldoon MF, Manuck SB, Matthews KA. Lowering cholesterol concentrations and mortality: a quantitative review of primary prevention trials. BJM 1990;301:390.
Peto R, Yusuf S, Collins R. Cholesterol-lowering trial results in their epidemiologic context. J Am Coll Cardiol 1991;17:111.
Oliver MF. Might treatment of hypercholesterolemia increase non-cardiac mortality? Lancet 1991;II:1529.
Canner PL, Berge KG, Wenger NK, Stamler J, Friedman L, Prineas RJ, Friedewald W. Fifteen year mortality in Coronary Drug Project patients: long term benefit with niacin. J Am Coll Cardiol 1986;8:1245.
Goodman DS. New guidelines for lowering blood cholesterol and coronary risk. In: Stokes J, Mancini M, eds. Hypercholes-terolemia: clinical and therapeutic implications. Atherosclerosis review. New York: Raven Press, 1988: 75–84.
Italian Consensus Conference. Abbassare la colesterolemia per ridurre la cardiopatia ischemica. Rome: National Research Council, 1986.
Frick MH, Elo O, Haapa K, Heinonen OP, Heinsalmi P, Helo P, Huttunen JK, Kaitaniemi P, Koskinen P, Manninen V, Maenpaa H, Malkonen M, Manttari M, Norola S, Paternack A, Pikkarainene J, Romo M, Sjoblom T, Nikkila EA. Helsinki Heart Study: primary prevention trial with gemfibrozil in middle-aged men with dyslipidemia. N Engl J Med 1987; 317:1237.
Endo A, Kuroda M, Tanzawa K. Competitive inhibition of 3-hydroxy-3-methyl-glutaryl coenzyme A reductase by ML236A and ML236B fungal metabolites having hypochelesterolemic activity. FEBS Lett 1976;72:323.
Grundy SM, Bilheimer DW. Inhibition of 3-hydroxy-3-methyl-glutaryl-CoA reductase by mevinolin in familial hypercholes-terolemia heterozygotes: effects on cholesterol balance. Proc Natl Acad Sci USA 1984;81:2538.
Bilheimer DW, Grundy SM, Brown MS, Goldstein JL. Mevinolin and colestipol stimulate receptor-mediated clearance of low density lipoprotein from plasma in familial hypercholesterolemia heterozygotes. Proc Natl Acad Sci USA 1983; 80:4124.
Ma PTS, Gil G, Sudhof TC, Bilheimer DW, Goldstein JL, Brown MS. Mevinolin, an inhibitor of cholesterol synthesis, induces mRNA for low density lipoprotein receptor in livers of hamsters and rabbits. Proc Natl Acad Sci USA 1986;83:8370.
Kovanen PT, Bilheimer DW, Goldstein JL, Jaramillo JJ, Brown MS. Regulatory role for hepatic low density lipoprotein receptors in vivo in the dog. Proc Natl Acad Sci USA 1981;78:1194.
Grundy SM. HMG-CoA reductase inhibitors for treatment of hypercholesterolemia. N Engl J Med 1988;319:24.
Reinher E, Rudling M, Stahlberg D, Berglund L, Ewerth S, Bjorkher E, Einarsson E, Angelin B. Influence of pravastatin, a specific inhibitor of HMG-CoA reductase, on hepatic metabolism of cholesterol. N Engl J Med 1990;323:224.
Gaddi A, Arca M, Ciarrocchi A, Fazio S, D'Alo G, Tiozzo R, Descovich GC, Calandra S. Pravastatin in heterozygous familial hypercholesterolemia: low density lipoprotein (LDL) cholesterol-lowering effect and LDL receptor activity on skin fibroblasts. Metabolism 1991;40:1074.
Lenaz G, ed Coenzyme Q. Chichester: Wiley, 1985.
Battino M, Fato R, Parenti Castelli G, Lenaz G. Coenzyme Q can control the efficiency of oxidative phosphorylation. Int J Tissue React 1990;12:13.
Lenaz G, Fato R, Castelluccio C, Battino M, Parenti Castelli G. Electron transport in the ubiquinone region of the mitochondrial respiratory chain. In: Kotyk A, Skoda J, Paces V, Kostka V, eds. Highlights of modern biochemistry. Zeist: VSP International Science Publishers; 1989; 873–881.
Folkers K, Littarru GP, Yamagami T., eds. Biochemical clinical aspects of coenzyme Q, vol b. North Holland: Elsevier, 1991.
Lenaz G, Barnabei O, Rabbi A, Battino M, eds. Highlights in ubiquinone research. London: Taylor and Francies 1990.
Lenaz G, Battino M, Castelluccio C, Fato R, Cavazzoni M, Rauchova H, Bovina C, Formiggini G, Parenti Castelli G. Studies on the role of ubiquinone in the control of the mitochondrial respiratory chain. Free Radic Res Commun 1990; 8:317.
Berliner JA, Territo MC, Sevanian A, Ramin S, Kim JA, Bamshad B, Esterson M, Fogelman AM. Minimally modified low density lipoprotein stimulates monocyte endothelial interactions. J Clin Invest 1990;85:1260.
Regnstrom J, Nilsson J, Tornvalle P, Landou C, Hamsten A. Susceptibility to low density lipoprotein oxidation and coronary atheroslerosis, in man. Lancet 1992;339:11833.
Stocker R, Bowry VW, Frei B. Ubiquinol-10 protects human low density lipoprotein more efficiently against lipid peroxidation than does alpha-tocopherol. Proc Natl Acad Sci USA 1991;88:1646.
Mohr D, Bowry VW, Stocker R. Dietary supplementation with CoQ10 results in increased levels of ubiquinol-10 within circulating lipoprotein and increased resistance of human low density lipoprotein to the initiation of lipid peroxidation. Biochim Biophys Acta 1992;1126:247.
Durrington PN. Hyperlipidemia: diagnosis and management. London: Wright, 1989.
Research Group ATS-RFS of the Italian National Research Council. Time trends of some cardiovascular risk factors in Italy. Am J Epidemiol 1987;126:95.
WHO-ERICA Research Group. The CHD risk-map of Europe. The 1st report of the WHO-ERICA project. Eur Heart J 1988;9:1.
Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low density lipoprotein cholesterol in plasma without the use of the preparative ultracentrifuge. Clin Chem 1972;18:499.
Röschlau P, Bernt E, Gruber R. Enzymatische Bestimmung des Gesamt-Cholesterins im Serum. Z Klin Chem Klin Biochem 1974;12:403.
Wahlefeld AW. Triglyceride determinations after enzymatic hydrolysis. In: Bergmayer HV, ed. Methods in enzymatic analysis. New York: Academic Press; 1974: 1831–1835.
Warnick GR, Cheung MC, Albers JJ. Comparison of current methods for high density lipoprotein cholesterol quantitation. Clin Chem 1979;25:596.
Takada M, Ikenoya S, Yuzuriha T, Katayama K. Studies on reduced and oxidized coenzyme Q (ubiquinones). The determination of oxidation reduction levels of coenzyme Q in mitochondria, microsome and plasma by high performance liquid chromatography. Biochim Biophys 1982;679:308.
Battino M, Bargossi AM, Fiorella PL, Lenaz G. Plasma and platelet coenzyme Q10 in trained athletes. Giorn It Chim Clin 1990;15:347.
Salganicoff L, Fukami MH. Energy metabolism of blood platelets. Arch Biochem Biophys 1972;153:725.
Lowry OH, Rosebrough NJ, Farr Al, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951; 193:265.
Kröger A. Determination of contents and redox states of ubiquinone and menaquinone. Methods Enzymol 1978;53:579.
Battino M, Bertoli E, Formiggini G, Sassi S, Gorini A, Vill RF, Lenaz G. Structural and functional aspects of the respiratory chain of synaptic and non-synaptic mitochondria derived from selected brain regions. J Bioenerg Biomembr 1991;23:345.
Bulpitt CJ. Cofidence intervals. Lancet 1987;II:494.
Gardner MJ, Altman DG. Confidence intervals rather thanP values: estimation rather than hypethesis testing. BMJ 1986: 292:746.
Statgraphics Statistical Graphics System. Rockville, Maryland, USA: Statistical Graphic Corporation, 1987.
Illingworth RD, Sexton JG. Hypocholesterolemic effects of mevinolin in patients with heterozygous familial hypercholes-terolemia. J Clin Invest 1984;74:1972.
Uauy R, Vega GL, Grundy SM, Bilheimer DM. Lovastatin therapy in receptor-negative homozygous familial hypercholes-terolemia: lack of effect on low-density lipoprotein concentrations or turnover. J Pediatr 1988;113:387.
Kamai M, Fujita T, Kanbe T, Sasaki K, Oshiba K, Otani S, Matsui-Yuasa I, Morisawa S. The distribution and content of ubiquinone in foods. Int J Vit Nutr Res 1986;57:57.
Ramasarma T. Natural occurrence and distribution of coenzyme Q: In: Lenaz G, ed. Coenzyme Q. Chichester: Wiley, 1985.
Ghirlanda G, Oradei A, Manto A, Lippa S, Uccioli L, Caputo S, Greco AV, Littarru GP. Evidence of plasma CoQ10-lowering effect by HMGCoA reductase inhibitors: a double blind, placebo-controlled study. J Clin Pharmacol 1993;33:226.
Mabuchi H, Haba T, Tatami R, Miyamoto S, Sakai Y, Wakasugi T, Watanabe A, Koizumi J, Takeda R. Effects of an inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A reductase on serum lipoproteins and ubiquinone-10 levels in patients with familial hypercholesterolemia. N Engl J Med 1981;305:478.
Gaddi A, Ciarrocchi A, Matteucci A, Rimondi S, Ravaglia G, Descovich GC, Sirtori CR. Dietary treatment for familial hypercholesterolemia— differential effects of dietary soy protein according to apo E phenotypes. Am J Clin Nutr 1991;53:1191.
Bargossi AM, Fiorella PL, Battino M, Bignami A, Lodi A, Urbini D, Sprovieri G. Average plasmatic and platelet CoQ10 level in thyroid disease: preliminary results. In: Folkers K, Littarru GP, Yamagami T, eds. Biomedical and clinical aspects of coenzyme Q. Amsterdam: Elsevier; 1991: 425–430.
Folkers K, Lansjoen P, Willis R, Richardson P, Xia LJ, Ye CQ, Tamagawa H Lovastatin decreases coenzyme Q levels in humans. Proc Natl Acad Sci USA 1990;87:8931.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bargossi, A.M., Battino, M., Gaddi, A. et al. Exogenous CoQ10 preserves plasma ubiquinone levels in patients treated with 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors. Int J Clin Lab Res 24, 171–176 (1994). https://doi.org/10.1007/BF02592449
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02592449