Abstract
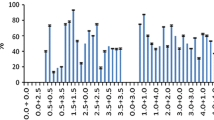
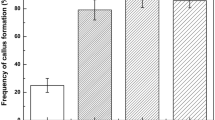
The frequency of plant regeneration from seed-derived Pokkali rice callus has been substantially increased. Four conclusions were drawn from the study: (1) Non-embryogenic callus consisting of elongated, highly-vacuolated cells did not produce regenerated plants. Embryogenic callus consisting of small, non-vacuolated cells produced somatic embryos and regenerated plants. (2) The numbers of plants could be markedly increased by optimizing a medium for embryogenic callus production and a second medium for plant regeneration from embryogenic callus. (3) The optimization of callus to medium volume ratio of 6.5 mg embryogenic callus per 1.0 ml of medium significantly increase plant production on regeneration medium. (4) A further significant increase was obtained by using regeneration medium previously conditioned for one or two weeks by optimal amounts of embryogenic callus. At present, the callus derived from a single seed in six months could theoretically be used in the seventh month to produce 127500 plants.
Similar content being viewed by others
References
Bhattacharya S, Chatterjee S, Biswas P, Mukherjee B (1981) Role of inoculum weight on physiology of growth and development of Corchorus olitorius and Nigella sativa tissue cultured in vitro. Indian J Exp Biol 19: 1030–1032.
Caplin SM (1963) Effect of initial size on growth of plant tissue cultures. Am J Bot 50: 91–94
Christianson ML, Warnick DA, Carlson PS (1983) A morphogenetically competent soybean suspension culture. Science 222: 632–634
Dunwell JM (1979) Anther culture in Nicotiana tabacum: The role of the culture vessel atmosphere in pollen embryo induction and growth. Jour Exp Botany 30: 419–428
Green CE, Armstrong CL, Anderson PC (1983) Somatic cell genetic system in corn. In: Fazelahmad A, Downey K, Schultz J, Voellmy RW (eds) Advances in gene technology: Molecular genetics of plants and animals. Academic Press
Heyser JW, Nabors MW, MacKinnon C, Dykes TA, DeMott KJ, Kautzman DC, Mujeeb-Kazi A (1984) Long-term, high freqency plant regeneration and the induction of somatic embryogenesis in callus cultures of wheat (Triticum aestivum L.). Z Pflanzenzuch (In Press)
Inoue M, Maeda E (1981) Stimulation of shoot bud and plantlet formation in rice callus cultures by two-step culture method using abscisic acid and kinetin. Japan Jour Crop Sci 50: 318–322
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia P1 15: 473–497
Nabors MW (1983) Increasing the salt and drought tolerance of crop plants. In: Randall DD (ed) Current topics in plant biochemistry and physiology. U Missouri, Columbia pp 165–184
Nabors MW, Heyser JW, Dykes TA, DeMott KJ, (1983) Long-duration, high-frequency plant regeneration from cereal tissue cultures. Planta 157: 385–391
Raghava Ram NV, Nabors MW (1983) Cytokinin mediated long-term, high-freqency plant regeneration in rice tissue cultures. Z Pflanzenphysiol 113: 315–323
Scowcroft WR, Larkin PL (1983) Somaclonal variation and genetic improvement of crop plants. In: Nugent J, O'Connor M (eds) Better crops for food. Pitman pp 177–193
Siriwardana S, Nabors MW (1983) Tyyptophan enhancement of somatic embryo-genesis in rice. Plant Physiol 73: 142–146
Skoog F, Miller W (1957) Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symp Soc Exp Biol 11: 118–131
Steward FC, Caplin SM (1954) The growth of carrot tissue explants and its relation to the growth factors present in coconut milk. I (A) The development of the quantitative method and the factors affecting the growth of carrot tissue explants. Ann Biol 30: 386–394
Street HE (1966) The nutrition and metabolism of plant tissue and organ cultures. In: Willmer EN (ed) Cells and tissues in culture. pp 533–620
Swamy BGL, Krishnamurthy KV (1981) On embryos and embryoids. Proc Indian Acad Sci 90: 401–411
Vajrabhaya M, Vajrabhaya T (1972) Variations in dendrobium arising in meristem. In: Ospina HM (ed) Proc 7th world orchid congress. Medellin, Columbia pp 232–243
Walker KA, Sato SJ (1981) Morphogenesis in callus tissue of Medicago sativa: the role of ammonium ion in somatic embryogenesis. Plant Cell Tissue Organ Culture 1: 109–121
Author information
Authors and Affiliations
Additional information
This research was supported by the Agency for International Development under Contract No. AID/DSAN-C-0273
Rights and permissions
About this article
Cite this article
Raghava Ram, N.V., Nabors, M.W. Plant regeneration from tissue cultures of Pokkali rice is promoted by optimizing callus to medium volume ratio and by a medium-conditioning factor produced by embryogenic callus. Plant Cell Tiss Organ Cult 4, 241–248 (1985). https://doi.org/10.1007/BF00040198
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00040198