Abstract
Aim
This study is designed to investigate the effects of tocotrienol-tocopherol mixed fraction (TTMF), vitamin C and combined TTMF-vitamin C supplementations on serum lipids and biochemical markers of inflammation and endothelial activation in hypercholesterolemic subjects in the low-risk category.
Materials and Methods
78 hypercholesterolaemic subjects (total cholesterol of ≥ 5.2 mmol/L and low-density lipoprotein 3.4 – 4.9 mmol/L) in the low cardiovascular risk category according to the NCEP-ATP3 criteria were recruited. They were randomized into four treatment combination groups for a period of twelve months; (1) receiving TTMF and vitamin C, (2) receiving TTMF and placebo, (3) receiving vitamin C and placebo, and (4) receiving placebo for both. Serum fasting lipid profiles and levels of high-sensitivity C-reactive protein, interleukin-6, tumour necrosis factor-ɑ, intercellular adhesion molecule, vascular cell adhesion molecule, E-selectin and homocysteine were measured at entry and multiple time points post-randomisation.
Results
There were no significant differences in percentage changes of lipid profiles and inflammatory markers between treated and placebo groups for either single or combined antioxidants supplementations.
Conclusion
TTMF, vitamin C and combined TTMF-vitamin C supplementations have neutral effects on lipid profiles and biochemical markers of inflammation and endothelial activation in low risk subjects, suggesting that they offer no added advantage in the low cardiovascular risk group.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The use of vitamins C and vitamin E as dietary supplements due to their supposed benefits in health and disease is widespread. Both are antioxidants, and as such is thought to reduce oxidative stress and its implications for the human body (Breilmann J et al.,2010). One of such implications is atherosclerosis, of which hypercholesterolaemia is a risk factor. Low density lipoproteins (LDL) that are elevated in hypercholesterolaemia are prone to oxidization (Palinski et al., 1989), thus initiating the cascade of atheroma formation by way of activating specific genes involved (Maziere and Maziere, 2009).
Elevated oxidized LDL (oxLDL) level is independently related to the risk of developing atherosclerosis and subsequent coronary heart disease (Toshima et al., 2000). Furthermore, it also contributes to atheroscle rotic plaque instability (Nishi et al., 2002; Okura et al., 2000), leading to thrombus formation and acute coronary events. Inflammation is considered to be the pathway by which oxLDL exerts its adverse cardiovascular effects (Bieghs et al., 2013; Shen et al., 2013). Additionally, oxLDL also promotes endothelial dysfunction via upregulation of its markers, such as the intercellular and vascular cell adhesion molecules (ICAM and VCAM, respectively) (Huang et al., 2013).
Efforts have been made to lower LDL levels by traditional medicine and/or medications to acceptable values for patients at risk of developing cardiovascular diseases, and the use of antioxidants to quench the oxidation of LDLs could prove to be a novel approach to this problem. However, research in this area is still not provided conclusive results to support their clinical use.
Tocotrienols and tocopherols are members of the vitamin E family. Tocotrienol has been proven to be a more potent antioxidant than tocopherols (Maniam et al., 2008). Furthermore, tocotrienol has been found to exert other effects outside of its antioxidant capabilities, such as cardioprotective (Das et al., 2007), neuroregenerative (Khanna et al., 2005) and anti-cancer properties (Aggarwal et al., 2010). It was also found to directly inhibit HMG-CoA reductase, the predominant enzyme in cholesterol metabolism (Khor and Ng, 2000). Vitamin C is an established antioxidant and has been demonstrated to reduce oxidative stress induced by multiple mechanisms (El-Gendy et al., 2010; Tsovolas et al., 2008). The combination of vitamins C and E has been shown to be beneficial in combating oxidative stress induced by intense exercise (Naziroglu et al., 2010) and X-ray (Kayan et al., 2009).
Due to the potential benefit of tocotrienol in hypercholesterolaemia, we investigated the effects of tocotrienol- tocopherol mixed fraction (TTMF) of palm oil and vitamin C, either alone or in combination, on lipid profile and biomarkers of inflammation and endothelial activation in patients at low risk of cardiovascular disease.
Materials and methods
Study design and endpoints
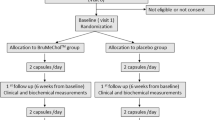
A randomized double blind placebo-controlled clinical trial was conducted in the Clinical Trial Center, Universiti Teknologi MARA in Selangor, Malaysia. Men and women ranged from 25 to 60 years old were screened for their cholesterol profile and assessed for their cardiovascular (CV) risk factors according to the National Cholesterol Education Plan-Adult Treatment Protocol 3 (NCEP-ATP3). Those with a LDL level of more than 3.4 mmol/l and one or less CV risk factor were recruited into the low risk category group. The exclusion criteria were: subjects with BMI >35kg/m2, fasting triglycerides >4.5mmol/l, diabetes mellitus, uncontrolled hypertension (systolic blood pressure >150mmHg), established coronary heart disease or peripheral vascular disease, chronic inflammatory disorders or severe disease with poor prognosis.
Using the Open Epi statistical calculator (www.openepi.com), a sample size of 16 subjects was needed to achieve a power of 80% to 95% confidence interval. A total of 78 subjects who gave informed consent were recruited and subsequently randomized into four groups: (1) TRF 80mg/day and vitamin C 500mg/day, (2) Vitamin C 500mg/day and placebo TRF, (3) TRF 80mg/day and placebo C, and (4) placebo of both. Patients were given clear instruction to take their supplementation either before or after dinner. Those who regularly consume antioxidants or already on lipid lowering medications were subjected to a 4- week washout period before starting on their allocated supplementations.
Blood sampling, anthropometric measurements and medical consultation on therapeutic lifestyle changes were done at the randomization stage. After that, subjects were called back for consultation and blood sampling at 2 weeks, 3 months, 6 months and 12 months. The endpoints recorded were the levels of the outlined biochemical parameters, whether they were improved as compared to baseline levels, and percentage of treated subjects improving their parameters as compared to placebo.
The experimental protocol complied with the Helsinki Declaration and was approved by the institutional Research Ethics Committee prior to the commencement of the study. All patients gave written informed consent for their participation in this study.
Materials
TTMF was supplied by Golden Hope Bioganic Sdn. Bhd., Malaysia with the following composition: alpha tocotrienol (59.2mg, 22.1%), beta tocotrienol (4.5mg, 1.7%), gamma tocotrienol (58.6mg, 21.9%), delta tocotrienol (37.6mg, 14.0%), alpha tocopherol (44.0mg 23.9%) and palm super olein (128.0mg, 23.9%). Commercially available Vitamin C was used (Flavettes®, ECM Pharma Sdn. Bhd., Malaysia). The placebo was produced as soft-gel capsules with similar excipient of Vitamin E (palm super olein) but without TTMF.
Biochemical parameters and analysis
During the randomization stage and subsequent visits, 20mls of venous blood was obtained from the subjects. Besides routine biochemical testing (fasting blood glucose; FBG and serum lipids; FSL), levels of high sensitivity C-reactive protein (hsCRP), interleukin- 6 (IL-6), tumour necrosis factor-ɑ (TNF-ɑ), intercellular adhesion molecule (ICAM), vascular cell adhesion molecule (VCAM), E-selectin and homocysteine were also determined from these samples. Total cholesterol (TC), triglycerides (TG), HDL-c and hsCRP were measured on an automated chemistry analyzer (Cobas Integra, Roche Diagnostics, Basel, Switzerland). IL-6 and TNF-ɑ were measured on automated immunoanalyser (Immulite 1000, Siemens Healthcare Diagnostics, USA). LDL-c concentration was derived by calculation using the Friedewald equation (Friedewald et al., 1972).
Statistical analysis
All data were entered and analyzed using Stata v11 software (StataCorp, Texas USA). All numerical variables were described as mean (SD) or median (IQR). Whereas categorical variables were summarized as frequencies and percentages. Normality of distribution was tested using the skewness and kurtosis test. Significance testing was done using the paired t-test for continuous variables and the chi-square test for categorical variables. P-values of less than 0.05 and 0.01 are considered significant and very significant, respectively.
Evaluation of the effects of TRF and vitamin C was done by combining treatment groups to increase the sample size of each treatment modality, with new groups designated A, B, C, D, E and F. Effects of TRF and/or vitamin C treatment on the various biochemical variables were evaluated by comparing group A (treatment groups 1 and 3) to group E (groups 2 and 4) to determine the effect of TRF, group B (groups 1 and 2) to group F (3 and 4) to determine the effect of vitamin C, and group C (groups 1, 2 and 3) to group D (group 4 only) to determine the effect of TRF and vitamin C in combination. Using these combinations, the sample size during each analysis of treatment effect is at least 38 subjects.
Results
Baseline characteristics
Table 1 shows the baseline characteristics of the patients recruited in this clinical trial according to their treatment group. From the table, the distribution of patients in the different treatment groups was similar as there were no significant differences in the baseline characteristics (Table 1 ).
Effect of supplements on fasting serum lipid profile:
Table 2 summarises the comparison between different treatment modalities in terms of the outcome of the fasting serum lipid parameters. For the TTMF (groups A vs E), there were trends of lowering of the plasma levels of TC, LDL, HDL and TG after the 12-month treatment as compared to baseline. Except for TG lowering (p = 0.05), the other observations were not statistically significant. Compared with TTMF placebo, a bigger percentage of TTMF treated subjects lowered their LDL, HDL and TG, with the percentage experiencing HDL-lowering reaching statistical significance (p = 0.05) (Table 2 ).
When combined with vitamin C (groups C vs D), TTMF modestly but significantly lowered TC (p = 0.02), HDL (p <0.01) and TG (p = 0.03) after 12 months of treatment compared to the baseline. A similar but insignificant trend was also observed for LDL. The percentage of subjects improving the combined treatment was higher compared to placebo for all the lipid parameters measured.
Effects of supplements on inflammatory mediators and markers for endothelial activation:
Table 3 and 4 summarise the comparison between the treatment groups in terms of assessing inflammation and endothelial activation. As for the inflammatory mediators (Table 3 ), subjects taking TTMF showed a tendency of lowering of all the inflammatory mediators after 12 months of treatment. When coupled with vitamin C, a similar trend was observed for IL6 and hsCRP. However, the percentage of treated subjects (TTMF and TTMF with vitamin C) improving their inflammatory mediator levels was smaller when compared to placebo over the same time period (Table 4 ).
TTMF treatment markedly increased homocysteine levels after 12 months when compared to placebo (p = 0.01). The combination of TTMF with vitamin C also showed a similar increment of homocysteine (p = 0.04). Among the additional endothelial activation biomarkers, 12-month treatment with TTMF showed a beneficial lowering trend only for VCAM. In terms of proportion of subjects improving their biomarkers on treatment, the percentage was smaller for TTMF treated subjects compared to placebo. When TTMF treatment is combined with vitamin C, a bigger percentage of subjects improved their biomarkers over the 12-month period, with the exception of E-selectin, where the percentage is significantly smaller (p = 0.05) (Table 4 ).
Discussion
After 12 months of treatment with TTMF, either alone or in combination, we found that despite the encouraging trends, TTMF had a neutral effect on the lipid parameters when compared to placebo. The utilisation of a mixture of tocotrienol and tocopherol, rather than a pure tocotrienol compound, could be the reason behind this observation. Although other human studies have demonstrated the benefit of this mixed fraction in lowering plasma lipids (Qureshi et al., 2001, 2002), the fraction of tocopherol in the mixture used by them yielded a lower tocopherol fraction, compared to our TTMF (8% vs 26%, respectively).
The effect of tocopherol on plasma lipids has been positively established (de Oliveira et al., 2011). However, its utilization in a mixture with tocotrienol has yielded conflicting results (Fu et al., 2014). One study suggested that tocopherol has a negative impact on the lipid lowering property of tocotrienols (Qureshi et al., 1995), via its stimulating effect on HMG-CoA reductase, the rate-limiting enzyme in the cholesterol synthesis pathway, thus attenuating the inhibitory effect exerted by tocotrienols (Qureshi et al., 1996). It was determined that in a mixed preparation of tocotrienol and tocopherol, 20% or more of the latter leads to attenuation of the former’s hypocholesterolaemic effects (Qureshi et al., 1995; Qureshi et al., 1991).
A worrying observation is the consequence of HDL lowering with TTMF in this study. HDL, which is a plasma cholesterol scavenger, is positively correlated with a reduction of adverse cardiovascular (CV) risk and CV mortality (Moradi et al., 2014). Whilst there is another study that reported a similar observation (Tan et al., 1991), most reported a neutral effect of tocotrienols on HDL levels (Baliarsingh et al., 2005; Mensink et al., 1999; Qureshi et al., 1995). Another study demonstrated that the same TTMF preparation was able to significantly increase plasma HDL after 6 months of treatment when compared to baseline levels (Chin et al., 2011). However, the same study showed that the HDL increment was only observed to be significantly better than placebo in the older age group (>50 years) compared to those aged between 35 – 49 years, a group where most of our subjects were located in.
Despite this observation with regard to the HDL levels, a bigger percentage of subjects on TTMF (alone or in combination with vitamin C) improved their lipid profiles. This trend is encouraging, and supports the notion that tocotrienols should be an adjunct therapy for attenuating hypercholesterolaemia. This is due to the inhibitory effect that tocotrienol has on HMG-CoA reductase (Khor and Ng, 2000), the same enzyme that is inhibited by the statin group of drugs. Owing to their synergistic interaction, the combination of lovastatin and tocotrienol-rich fraction (TRF) has been shown to be effective in lowering plasma lipid parameters (Qureshi et al., 2001).OxLDL predisposes to inflammation (Bieghs et al., 2013) and endothelial dysfunction (Huang et al., 2013). In our study, we demonstrated that TTMF treatment had a neutral effect on hsCRP and TNF-ɑ, and a tendency for lowering of IL6 levels, thus proving to be potentially beneficial in quenching oxLDL-mediated inflammation. Similarly, one study has reported that TRF has a neutral effect on CRP and IL6 in haemodialysis patients (Daud et al., 2013), but another reported that the level of CRP precursor protein was down-regulated in healthy individuals treated with TRF (Heng et al., 2013).
TTMF was also found to have a neutral effect on biomarkers of endothelial activation in the current study. Even though many in-vitro studies have reported tocotrienol’s beneficial effect on adhesion molecules expression (Ahn et al., 2007; Naito et al., 2005; Theriault et al., 2002), ours is the only study that evaluated its role in human. However, the homocysteine elevation seen with TTMF treatment when compared to the baseline is in disagreement with a study in animals, which have demonstrated a beneficial lowering of homocysteine with tocotrienol treatment (Norsidah et al., 2013). One study in humans yielded a neutral effect of tocopherol, rather than tocotrienol, on homocysteine levels (Breilmann et al., 2010). Although the effect observed here is minimal, due to the correlation of homocysteine to cardiovascular risk (Nygård et al., 1995), further studies should aim to confirm this finding and look at the possible mechanisms involved, if any.
Conclusion
Treatment for 12- months with TTMF, either alone or in combination with vitamin C supplementations have neutral effects on lipid profiles and biochemical markers of inflammation and endothelial activation in low risk subjects, suggesting that they offer no added advantages in the low cardiovascular risk group. The utilization of TTMF with its high tocopherol fraction (26%) has probably impeded the improvement observed, due to antagonistic interaction between tocopherols and tocotrienols, although worrying, the negative impact of TTMF on plasma HDL and homocysteine is quite minimal. In the future, more studies will be needed to assess the effect of tocotrienols in human, especially on biomarkers for endothelial activation or dysfunction.
References
Aggarwal, B.B., Sundaram, C., Prasad, S., and Kannappan, R. (2010). Tocotrienols, the vitamin E of the 21st century: its potential against cancer and other chronic diseases. Biochem Pharmacol 80, 1613–1631.
Ahn, K.S., Sethi, G., Krishnan, K., and Aggarwal, B.B. (2007). Gammatocotrienol inhibits nuclear factor-kappaB signaling pathway through inhibition of receptor-interacting protein and TAK1 leading to suppression of antiapoptotic gene products and potentiation of apoptosis. The Journal of biological chemistry 282, 809–820.
Baliarsingh, S., Beg, Z.H., and Ahmad, J. (2005). The therapeutic impacts of tocotrienols in type 2 diabetic patients with hyperlipidemia. Atherosclerosis 182, 367–374.
Bieghs, V., Walenbergh, S.M., Hendrikx, T., van Gorp, P.J., Verheyen, F., Olde Damink, S.W., Masclee, A.A., Koek, G.H., Hofker, M.H., Binder, C.J., et al. (2013). Trapping of oxidized LDL in lysosomes of Kupffer cells is a trigger for hepatic inflammation. Liver international: official journal of the International Association for the Study of the Liver 33, 1056–1061.
Breilmann, J., Pons-Kühnemann, J., Brunner, C., Richter, M., and Neuhäuser-Berthold, M. (2010). Effect of Antioxidant Vitamins on the Plasma Homocysteine Level in a Free-Living Elderly Population. Annals of Nutrition and Metabolism 57, 177–182.
Chin, S.F., Ibahim, J., Makpol, S., Abdul Hamid, N.A., Abdul Latiff, A., Zakaria, Z., Mazlan, M., Mohd Yusof, Y.A., Abdul Karim, A., and Wan Ngah, W.Z. (2011). Tocotrienol rich fraction supplementation improved lipid profile and oxidative status in healthy older adults: A randomized controlled study. Nutrition & metabolism 8, 42.
Das, S., Nesaretnam, K., and Das, D.K. (2007). Tocotrienols in cardioprotection. Vitam Horm 76, 419–433.
Daud, Z.A., Tubie, B., Sheyman, M., Osia, R., Adams, J., Tubie, S., and Khosla, P. (2013). Vitamin E tocotrienol supplementation improves lipid profiles in chronic hemodialysis patients. Vascular health and risk management 9, 747–761.
de Oliveira, A.M., Rondo, P.H., Luzia, L.A., D’Abronzo, F.H., and Illison, V.K. (2011). The effects of lipoic acid and alpha-tocopherol supplementation on the lipid profile and insulin sensitivity of patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled trial. Diabetes research and clinical practice 92, 253–260.
El-Gendy, K.S., Aly, N.M., Mahmoud, F.H., Kenawy, A., and El-Sebae, A.K. (2010). The role of vitamin C as antioxidant in protection of oxidative stress induced by imidacloprid. Food Chem Toxicol 48, 215–221.
Friedewald, W.T., Levy, R.I., and Fredrickson, D.S. (1972). Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clinical chemistry 18, 499–502.
Fu, J.Y., Che, H.L., Tan, D.M., and Teng, K.T. (2014). Bioavailability of tocotrienols: evidence in human studies. Nutrition & metabolism 11, 5.
Heng, E.C., Karsani, S.A., Abdul Rahman, M., Abdul Hamid, N.A., Hamid, Z., and Wan Ngah, W.Z. (2013). Supplementation with tocotrienol-rich fraction alters the plasma levels of Apolipoprotein A-I precursor, Apolipoprotein E precursor, and C-reactive protein precursor from young and old individuals. European journal of nutrition 52, 1811–1820.
Huang, C.S., Lin, A.H., Liu, C.T., Tsai, C.W., Chang, I.S., Chen, H.W., and Lii, C.K. (2013). Isothiocyanates protect against oxidized LDLinduced endothelial dysfunction by upregulating Nrf2-dependent antioxidation and suppressing NFkappaB activation. Molecular nutrition & food research 57, 1918–1930.
Kayan, M., Naziroglu, M., Celik, O., Yalman, K., and Koylu, H. (2009). Vitamin C and E combination modulates oxidative stress induced by X-ray in blood of smoker and nonsmoker radiology technicians. Cell Biochem Funct 27, 424–429.
Khanna, S., Roy, S., Slivka, A., Craft, T.K., Chaki, S., Rink, C., Notestine, M.A., DeVries, A.C., Parinandi, N.L., and Sen, C.K. (2005). Neuroprotective properties of the natural vitamin E alpha-tocotrienol. Stroke 36, 2258–2264.
Khor, H.T., and Ng, T.T. (2000). Effects of administration of alphatocopherol alphatocopherol and tocotrienols on serum lipids and liver HMG CoA reductase activity. Int J Food Sci Nutr 51 Suppl, S3–11.
Maniam, S., Mohamed, N., Shuid, A.N., and Soelaiman, I.N. (2008). Palm tocotrienol exerted better antioxidant activities in bone than alphatocopherol. Basic Clin Pharmacol Toxicol 103, 55–60.
Maziere, C., and Maziere, J.C. (2009). Activation of transcription factors and gene expression by oxidized low-density lipoprotein. Free Radic Biol Med 46, 127–137.
Mensink, R.P., van Houwelingen, A.C., Kromhout, D., and Hornstra, G. (1999). A vitamin E concentrate rich in tocotrienols had no effect on serum lipids, lipoproteins, or platelet function in men with mildly elevated serum lipid concentrations. The American journal of clinical nutrition 69, 213–219.
Moradi, H., Streja, E., Kashyap, M.L., Vaziri, N.D., Fonarow, G.C., andKalantar-Zadeh, K. (2014). Elevated high-density lipoprotein cholesterol and cardiovascular mortality in maintenance hemodialysis patients. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association.
Naito, Y., Shimozawa, M., Kuroda, M., Nakabe, N., Manabe, H., Katada, K., Kokura, S., Ichikawa, H., Yoshida, N., Noguchi, N., et al. (2005). Tocotrienols reduce 25-hydroxycholesterol-induced monocyte-endothelial cell interaction by inhibiting the surface expression of adhesion molecules. Atherosclerosis 180, 19–25.
Naziroglu, M., Kilinc, F., Uguz, A.C., Celik, O., Bal, R., Butterworth, P.J., and Baydar, M.L. (2010). Oral vitamin C and E combination modulates blood lipid peroxidation and antioxidant vitamin levels in maximal exercising basketball players. Cell Biochem Funct 28, 300–305.
Nishi, K., Itabe, H., Uno, M., Kitazato, K.T., Horiguchi, H., Shinno, K., and Nagahiro, S. (2002). Oxidized LDL in carotid plaques and plasma associates with plaque instability. Arterioscler Thromb Vasc Biol 22, 1649–1654.
Norsidah, K.-Z., Asmadi, A.Y., Azizi, A., Faizah, O., and Kamisah, Y. (2013). Palm Tocotrienol-Rich Fraction Improves Vascular Proatherosclerotic Changes in Hyperhomocysteinemic Rats. Evidence-Based Complementary and Alternative Medicine 2013, 10.
Nygård, O., Vollset, S., Refsum, H., and et al. (1995). Total plasma homocysteine and cardiovascular risk profile: The hordaland homocysteine study. JAMA 274, 1526–1533.
Okura, Y., Brink, M., Itabe, H., Scheidegger, K.J., Kalangos, A., and Delafontaine, P. (2000). Oxidized low-density lipoprotein is associated with apoptosis of vascular smooth muscle cells in human atherosclerotic plaques. Circulation 102, 2680–2686.
Palinski, W., Rosenfeld, M.E., Yla-Herttuala, S., Gurtner, G.C., Socher, S.S., Butler, S.W., Parthasarathy, S., Carew, T.E., Steinberg, D., and Witztum, J.L. (1989). Low density lipoprotein undergoes oxidative modification in vivo. Proc Natl Acad Sci U S A 86, 1372–1376.
Qureshi, A.A., Bradlow, B.A., Brace, L., Manganello, J., Peterson, D.M., Pearce, B.C., Wright, J.J., Gapor, A., and Elson, C.E. (1995). Response of hypercholesterolemic subjects to administration of tocotrienols. Lipids 30, 1171–1177.
Qureshi, A.A., Pearce, B.C., Nor, R.M., Gapor, A., Peterson, D.M., and Elson, C.E. (1996). Dietary alpha-tocopherol attenuates the impact of gamma-tocotrienol on hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in chickens. The Journal of nutrition 126, 389–394.
Qureshi, A.A., Qureshi, N., Hasler-Rapacz, J.O., Weber, F.E., Chaudhary, V., Crenshaw, T.D., Gapor, A., Ong, A.S., Chong, Y.H., Peterson, D., et al. (1991). Dietary tocotrienols reduce concentrations of plasma cholesterol, apolipoprotein B, thromboxane B2, and platelet factor 4 in pigs with inherited hyperlipidemias. The American journal of clinical nutrition 53, 1042 S-1046 S.
Qureshi, A.A., Sami, S.A., Salser, W.A., and Khan, F.A. (2001). Synergistic effect of tocotrienol-rich fraction (TRF(25)) of rice bran and lovastatin on lipid parameters in hypercholesterolemic humans. The Journal of nutritional biochemistry 12, 318–329.
Qureshi, A.A., Sami, S.A., Salser, W.A., and Khan, F.A. (2002). Dosedependent suppression of serum cholesterol by tocotrienol-rich fraction (TRF25) of rice bran in hypercholesterolemic humans. Atherosclerosis 161, 199–207.
Shen, Y., Yang, T., Guo, S., Li, X., Chen, L., Wang, T., and Wen, F. (2013). Increased serum ox-LDL levels correlated with lung function, inflammation, and oxidative stress in COPD. Mediators of inflammation 2013, 972347.
Tan, D.T., Khor, H.T., Low, W.H., Ali, A., and Gapor, A. (1991). Effect of a palm-oil-vitamin E concentrate on the serum and lipoprotein lipids in humans. The American journal of clinical nutrition 53, 1027 S-1030 S.
Theriault, A., Chao, J.T., and Gapor, A. (2002). Tocotrienol is the most effective vitamin E for reducing endothelial expression of adhesion molecules and adhesion to monocytes. Atherosclerosis 160, 21–30.
Toshima, S., Hasegawa, A., Kurabayashi, M., Itabe, H., Takano, T., Sugano, J., Shimamura, K., Kimura, J., Michishita, I., Suzuki, T., et al. (2000). Circulating oxidized low density lipoprotein levels. A biochemical risk marker for coronary heart disease. Arterioscler Thromb Vasc Biol 20, 2243–2247.
Tsovolas, K., Iliodromitis, E.K., Andreadou, I., Zoga, A., Demopoulou, M., Iliodromitis, K.E., Manolaki, T., Markantonis, S.L., and Kremastinos, D.T. (2008). Acute administration of vitamin C abrogates protection from ischemic preconditioning in rabbits. Pharmacol Res 57, 283–289.
Cite this article as:
Osman, M., Rahman, T., Muid, S., Haron, H., Ismail, T., Ramli, A., Abdulrahman, A., & Nawawi, H. (2016). Effects of adding tocotrienol-tocopherol mixed fraction and vitamin C on inflammatory status in hypercholesterolaemic patients in the low coronary risk category. Biomedical Research And Therapy, 3(3), 557-566.
Acknowledgement
The authors would like to express appreciation to the Ministry of Higher Education, Malaysia for the financial support given under the Intensified Research for Prioritized Research (IRPA) code 06-02-02-0026-PR 001406-03 for the grant awarded to corresponding author, Universiti Teknologi MARA for the laboratory facilities and Sime Darby Bioganic Sdn Bhd for providing TriE.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License (CC-BY 4.0) which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit https://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Osman, M., Rahman, T., Muid, S. et al. Effects of adding tocotrienol-tocopherol mixed fraction and vitamin C on inflammatory status in hypercholesterolaemic patients in the low coronary risk category. Biomed Res Ther 3, 13 (2016). https://doi.org/10.7603/s40730-016-0013-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.7603/s40730-016-0013-9