Abstract
Retinoic acid which belongs to the retinoid class of chemical compounds is an important metabolite of vitamin A in diets. It is currently understood that retinoic acid plays important roles in cell development and differentiation as well as cancer treatment. Lung, prostate, breast, ovarian, bladder, oral, and skin cancers have been demonstrated to be suppressed by retinoic acid. Our results also show that low doses and high doses of retinoic acid may respectively cause cell cycle arrest and apoptosis of cancer cells. Also, the common cell cycle inhibiting protein, p27, and the new cell cycle regulator, Cdk5, are involved in retinoic acid’s effects. These results provide new evidence indicating that the molecular mechanisms of/in retinoic acid may control cancer cells’ fates. Since high doses of retinoic acid may lead to cytotoxicity, it is probably best utilized as a potential supplement in one’s daily diet to prevent or suppress cancer progression. In this review, we have collected numerous references demonstrating the findings of retinoic acid in melanoma, hepatoma, lung cancer, breast cancer, and prostate cancer. We hope these observations will shed light on the future investigation of retinoic acid in cancer prevention and therapy.
Similar content being viewed by others
1. Introduction
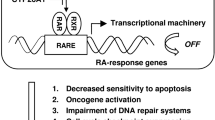
Vitamins are nutrients essential for the body’s growth, differentiation, development, and protection., Vitamin A is especially important because it can’t be synthesized by animals and must be supplied from a diet that includes plants [1]. There are many derivatives of vitamin A, including β-carotene, retinol, retinal, isotetrinoin, and retinoic acid. Treatment using retinoic acid was approved by the U.S. Food and Drug Administration for lymphoma [2] and leukemia [3]. Since retinoic acid is known to be effective in treating cancer, its basic structure has been well identified. All of the retinoids, including retinoic acid, are comprised of three units: a bulky hydrophobic region, a linker region, and a polar region (carboxylic acid terminus). There are many compounds derived from the above basic structure, and these compounds are collectively called retinoids [1]. Due to the efficiency of natural retinoids in cancer treatment, synthetic retinoids have been generated and investigated. In anti-cancer research, retinoic acid has been investigated and found to inhibit the markers of cell proliferation, such as cyclin D1 and human telomerase reverse transcriptase (hTERT), and growth factor, such as epidermal growth factor receptor (EGFR) and vascular endothelial growth factor (VEGF) [1]. The biological functions inhibited by retinoic acid include tumor growth, angiogenesis, and metastasis [1]. In addition, retinoic acid has also been found to regulate mitochondrial permeability, death receptors, ubiquitination, and reactive oxygen species, etc. [4]. It is believed that the inhibitory effects of retinoic acid are achieved through activating the retinoic acid receptor (RAR) or retinoic X receptor (RXR). RAR and RXR form heterodimers and function after ligand binding. To turn on downstream gene expression, RAR and RXR shuttle into cell nuclei and bind to the retinoic acid response elements (RARE), which are located in the 5’-region of retinoic acid downstream genes [5]. The activation of the above classical pathway will lead to cell differentiation, arrest, and eventually apoptosis [6]. In addition to the above classic pathway, retinoic acid may also regulate the downstream gene expression through modulating other transcription factors, such as NF-κB, IFN-γ, TGF-β, MAPK, and even chromatin remodeling [4]. RARs/RXRs heterodimerize with other receptors and regulate these partner receptors’ signaling, including non-classical or non-genomic pathways [7]. Sometimes, these partner receptors have opposite functions to RARs/RXRs. The latest finding of retinoic acid is the regulation of stem cell differentiation [8]. Ying et al. found that retinoic acid induces the expression of lineage-specific differentiation markers Tujl and GFAP and reduces the expression of neural stem cell markers such as CD133,
Msi-1, nestin, and Sox-2 [8]. In their expression microarray analysis, retinoic acid-affected pathways include retinoid signaling and metabolism, cell adhesion, cell-matrix interaction and cytoskeleton remodeling. Notch pathway down-regulation was also reported by retinoic acid-induced HES and HEY inhibition [8].
Although there are several lines of evidence indicating the effects and mechanisms of retinoic acid in cancer therapy, the chemo-prevention and therapeutic application of retinoic acid remain controversial. Here, this mini-review article demonstrates an overview of the research to date in the field of retinoic acid application and therapy to various types of cancer. The hope is that this review may impart readers with a better understanding of the research history of retinoic acid as well as guide the future direction in the field.
2. Retinoic acid and melanoma
Retinoic acid has been found to have inhibitory effects on growth of murine melanomas [9] and colony formation of human melanomas [10]. Activations of cyclic AMP-dependent protein kinase and sialyltransferase have also been found to involve the effects of retinoic acid [7, 11]. On the other hand, the modulation of melanoma cell adhesion to basement membrane components has been shown to be affected by retinoic acid treatment [12, 13]. Intercellular adhesion molecule gene I (ICAM-1) is transcriptionally regulated by retinoic acid in melanoma cells [14]. Retinoic acid has also been indicated to inhibit highly metastatic B16F10 melanoma cells by down-regulating the cell surface integrin receptors against extracellular matrix proteins, specifically laminin and vitronectin [15]. Since the formation of melanoma is correlated to radiation, retinoic acid has been found to modify the radio-sensitivity and recovery from X-ray damage in vitro [16]. Notably, the induction of protein kinase C in mouse melanoma cells was identified by retinoic acid treatment [17]. Ultraviolet irradiation may deplete cellular retinol and alter the metabolism of retinoic acid in cultured human keratinocytes and melanocytes [18]. In addition to inhibiting growth, retinoic acid has been found to inhibit human melanoma tumor cell invasion [19]. Epidermal growth factor receptor (EGFR) is a crucial player in epithelial cells in both growth and migration/invasion. Yongshan et al. discovered that EGFR expression was regulated by retinoic acid treatment [20]. In 1993, the combination treatment of interferon-α and retinoic acid was first believed to have significant therapeutic effect on melanoma by clinical examination [21]. The antitumor effect of green tea polyphenol on melanoma was enhanced by retinoic acid [22]. Interestingly, the differential regulation of tyrosinase activity in the skin of white and black individuals in vivo by retinoic acid was demonstrated [23]. In regards to drug delivery improvement, retinoic acid was encapsulated by liposome to treat melanoma cells and was then implanted onto C57BL/6 mice, with result of metastatic ability being efficiently suppressed [24]. A hyaluronic acid-based multifunctional nano-carrier was also used to deliver retinoic acid in cancer treatment tests [25]. All things considered, Retinoic acid seems to be a promising treatment for melanoma and more details will be investigated in the future to strengthen the basis of its mechanism.
3. Retinoic acid and hepatoma
Hepatoma is a serious form of cancer in Asia. It has been found that retinoic acid may directly cause the increase in protein synthesis of transferrin and albumin in Hep3B cells [26]. Since hepatitis virus infection is important to hepatoma formation, Hsu et al. demonstrate that retinoic acid may regulate the gene expression of hepatitis B virus surface antigen (HBsAg) in hepatoma cells [27]. Much cancer research focuses on the involvement of topoisomerase in cancer cell growth. Tsao et al. has reported that retinoic acid represses the expression of topoisomerase II in Hep3B cells [28]. The most current research of retinoic acid has used the model of short-term treatment and therefore been questioned in clinical therapy. However, Hsu et al. have demonstrated that long-term treatment with retinoic acid (30 days) may lead to suppression of the tumorigenicity of human hepatoma cells [29]. Furthermore, apoptosis of hepatoma cells was found after retinoic acid treatment and prevented by serum albumin and enhanced by lipoidol [30]. In addition, p21 induction and cdc2 activation are found to involve retinoic acid-induced hepatoma apoptosis [31]. Since retinoic acid may cause detachment of cancer cells under serum starvation, proteolysis of integrin α5 and β1 subunits were found in hepatoma cells [32]. The latest research indicates that retinoic acid may cooperate with arsenic to induce apoptosis and modulate the intracellular concentration of calcium in hepatoma cells [33]. Additionally, the retinoic acid receptor-related receptor α is believed to be a prognostic marker for hepatoma [34]. Taken together, these observations elucidate the fact that retinoic acid is indeed a potential compound to suppress hepatoma growth and cause hepatoma apoptosis. It’s also possible that retinoic acid can work as a helper that cooperates with other treatments and attacks hepatoma.
4. Retinoic acid and lung cancer
The incidence and mortality rates of lung cancer make this disease an important topic in cancer research. Since the relevant contribution of retinoic acid in cancers was discovered, there have been numerous studies demonstrating the effects of retinoic acid in lung cancer progression. At first, Hsu et al. found retinoic acid-mediated G1 arrest to be associated with induction of p27 and Cdk3 inhibition in lung squamous carcinoma cells [35]. In C57BL/6 mice model, retinoic acid was encapsulated and inhibited lung cancer metastasis [36]. Syndecan-1 is a proteoglycan that mediates cell-cell adhesion and prevents invasion in epithelial cells. Retinoic acid may increase syndecan-1 expression to block invasion/metastasis of lung cancer [37]. Notably, retinoic acid has been found to reduce chemotherapy-induced neuropathy in an animal model as well as patients with lung cancer [38]. These results show the relevance of retinoic acid in lung cancer treatment.
5. Retinoic acid and breast cancer
The application of retinoic acid in breast cancer treatment was first mentioned in 1970’s [39]. A retinoic acid-binding protein is believed to be an important factor in the progression of breast cancer [40, 41]. The latest report indicates that the sensitivity of retinoic acid in triple negative breast cancer cell lines may be restored by other treatment, such as curcumin [42]. Aldehyde dehydrogenase 1A3 (ALDH1A3) influences breast cancer progression via differential retinoic acid signaling [43]. Besides the above, a different type of protein kinase C was also found to involve the induction of the retinoic acid system in breast cancer [44]. Notably, retinoic acid may induce re-differentiation of early transformed breast epithelial cells [45], suggesting the preventive role retinoic acid plays with respect to breast cancer. Kamal et al. drew attention to the effect of retinoic acid by proteomic analysis in breast cancer cell lines [46]. The amplification of the retinoic acid receptor α (RARα) and retinoic acid sensitivity were found to correlate to breast cancer progression [47]. Retinoic acid can impair estrogen signaling in breast cancer cells by interfering with the activation of LSD1 via protein kinase A [48]. Retinoic acid was also found to reduce breast cancer growth and lung metastasis [49]. The procoagulant activity of breast cancer cells was reported to be modulated by retinoic acid [50]. Interestingly, microRNA-21 was found to be induced by retinoic acid in breast cancer, which suggests the biological correlation and molecular targets in breast cancer [51]. In addition, retinoic acid may inhibit aromatase activation and expression, which indicates that the estrogen supply inside breast cancer cells is insufficient to maintain cancer cell growth [52]. In addition to growth inhibition, retinoic acid is able to down-regulate MMP-9 by modulating its regulatory molecules and therefore impacts the invasion ability of breast cancer cells [53]. Additionally, retinoic acid may inhibit telomerase activation through inducing histone deacetylation in estrogen receptor-negative breast cancer cells [54]. Importantly, Hau et al. elucidated the genomic antagonism between retinoic acid and estrogen signaling in breast cancer and published their findings in the journal, Cell [55]. Their article shows the critical and solid thought of retinoic acid application to breast cancer. Since HOXA5 plays a role in apoptosis of breast cancer cells, retinoic acid was reported to regulate HOXA5 through RAR-β [56]. Cell cycle control gene, Btg2, is believed to be a direct target for RAR signaling in breast cancer cells [57]. Moreover, retinoic acid may sensitize breast cancer cells to taxol through down-regulation of survivin and promote the aberrant mitotic progression that causes apoptosis [58]. Although a lot of evidence demonstrates the effectiveness of the application of retinoic acid to breast cancer, combination treatments with other effective compounds (such as tomaxifen, taxol, and interferone) has been proposed and is currently utilized.
6. Retinoic acid and prostate cancer
Just like breast cancer, the history of retinoic acid treatment for prostate cancer has a strong history going back to the 1980’s. Researchers’ attention then was focused on the retinoic acid receptor in the study of prostate cancer cells [59]. The effects of retinoic acid on the growth and morphology of a prostate cancer cell line was first investigated [60]. After that, the binding proteins of retinoic acid were identified [61-63]. Since prostate cancer cells are eager to require androgen supplement in the early stages of the disease, 5α-reductase becomes important to provide potent androgens. Retinoic acid was found to inhibit 5α-reductase and therefore became a possible treatment for prostate cancer [64, 65]. Notably, the relationship between retinoic acid and prostate cancer growth was officially mentioned by Whelan [66]. Fong et al. demonstrated that retinoic acid at 10 μM may cause inhibition of androgen-dependent prostate cancer cell growth but may cause stimulation when the concentration is 0.01 μM [67]. The growth of androgen-independent prostate cancer cells is also suppressed by retinoic acid [68]. Extracellular matrices were also found to be regulated by retinoic acid [69, 70]. Specifically, retinoic acid has been found to activate the tumor suppressor, Rb, and decline androgen receptor proteins, thereby causing apoptosis of prostate cancer cells [71]. Interestingly, retinoic acid has been reported to interact with androgen in prostate cancer cells, which affects cell proliferation and expressions of retinoic acid receptor and epidermal growth factor receptor [72]. There is some research that demonstrates that the retinoid X receptor (RXR) might play important roles in tumorigenesis of prostate [73, 74]. RXR was also found to involve retinoic acid-induced inhibition of androgen receptor [75]. Hypermethylation of the retinoid acid receptor β is believed to be a prognostic marker in prostate cancer [76, 77]. Notably, the retinoic acid synthesis gene aldehyde dehydrogenase, ALDH1A2, is believed to be a candidate tumor suppressor in prostate cancer [78], which is similar to breast cancer as described above. More solid evidence has been provided by Huss et al., in which they have indicated that retinoic acid may slow the progression of prostate cancer and promote apoptosis of cancer cells [79]. In addition, retinoic acid was found to regulate the formation and degradation of gap junctions in prostate cancer cells [80]. Also, retinoic acid may inhibit the proliferation of prostate cancer cells through reducing the methylation level of the HOXB13 gene [81] and the Cdk5-dependent p27 expression [82]. Instead of growth inhibition, high doses of retinoic acid may cause apoptosis of prostate cancer cells though p35 cleavage and Cdk5 overactivation [83]. Although clinical trials have not shown strong evidence indicating that retinoic acid is an effective drug for prostate cancer [84, 85], more and more effort has been put toward retinoic acid research as it relates the nutritional supply and combination therapies with respect to prostate cancer.
7. Conclusion
Retinoic acid has been investigated extensively for its use in treating different forms of cancer not only in prevention but also in treatment. In this review, we described the research and applications of retinoic acid in melanoma, hepatoma, lung cancer, breast cancer, and prostate cancer. As a nutrient, retinoic acid may be obtained from either through the daily metabolization of plants in a balanced diet or through vitamin supplements. Under normal circumstances in the body, retinoic acid does preventive work against cancer formation. After cancer formation, retinoic acid becomes an attacker to cancer cells, one that blocks their growth and division and also triggers their differentiation and death through specific pathways. Furthermore, retinoic acid has been proven to cooperate with other effective cancer therapeutic drugs against cancer progression. Retinoic acid becomes a helper to chemo-therapeutic agents, a helper which may decrease both the dosages of these chemo-therapeutic agents required and their side-effects. This may relieve patients’ pain from chemotherapy and improve patients’ quality of life. From these points of view, although there has been a long history and no small amount of controversy regarding retinoic acid application in cancer treatment, it’s still worthwhile to continue research and place future effort toward gaining a more complete understanding of the application of retinoic acid in cancer treatment.
Acknowledgements
The study was supported by Taiwan Ministry of Science and Technology with regular grant (NSC 101-2320-B-005-004-MY3 to H.L.) and 2014 New Partnership Program for the Connection to the Top Labs in the World (103-2911-I-005-507/104-2911-I-005-501 to H.L.).
References
Alizadeh F, Bolhassani A, Khavari A, Bathaie SZ, Naji T, Bidgoli SA. Retinoids and their biological effects against cancer. Int Immunopharmacol 2014; 18: 43–9.
Duvic M, Hymes K, Heald P, Breneman D, Martin AG, Myskowski P, et al. Bexarotene is effective and safe for treatment of refractory advanced-stage cutaneous T-cell lymphoma: multinational phase II-III trial results. J Clin Oncol 2001; 19: 2456–71.
Tallman MS, Andersen JW, Schiffer CA, Appelbaum FR, Feusner JH, Ogden A, et al. All-trans-retinoic acid in acute promyelocytic leukemia. N Engl J Med 1997; 337: 1021–8.
Connolly RM, Nguyen NK, Sukumar S. Molecular pathways: current role and future directions of the retinoic acid pathway in cancer prevention and treatment. Clin Cancer Res 2013; 19: 1651–9.
Bushue N, Wan YJ. Retinoid pathway and cancer therapeutics. Adv Drug Deliv Rev 2010; 62: 1285–98.
Tang XH, Gudas LJ. Retinoids, retinoic acid receptors, and cancer. Annu Rev Pathol 2011; 6: 345–64.
Deutsch V, Lotan R. Stimulation of sialyltransferase activity of melanoma cells by retinoic acid. Exp Cell Res 1983; 149: 237–45.
Ying M, Wang S, Sang Y, Sun P, Lal B, Goodwin CR, et al. Regulation of glioblastoma stem cells by retinoic acid: role for Notch pathway inhibition. Oncogene 2011; 30: 3454–67.
Lotan R, Giotta G, Nork E, Nicolson GL. Characterization of the inhibitory effects of retinoids on the in vitro growth of two malignant murine melanomas. J Natl Cancer Inst 1978; 60: 1035–41.
Meyskens FL, Jr., Salmon SE. Inhibition of human melanoma colony formation by retinoids. Cancer Res 1979; 39: 4055–7.
Ludwig KW, Lowey B, Niles RM. Retinoic acid increases cyclic AMP-dependent protein kinase activity in murine melanoma cells. J Biol Chem 1980; 255: 5999–6002.
Edward M, Gold JA, MacKie RM. Modulation of melanoma cell adhesion to basement membrane components by retinoic acid. J Cell Sci 1989; 93 (Pt 1): 155–61.
Wang Z, Cao Y, D’Urso CM, Ferrone S. Differential susceptibility of cultured human melanoma cell lines to enhancement by retinoic acid of intercellular adhesion molecule 1 expression. Cancer Res 1992; 52: 4766–72.
Cilenti L, Toniato E, Ruggiero P, Fusco C, Farina AR, Tiberio A, et al. Transcriptional modulation of the human intercellular adhesion molecule gene I (ICAM-1) by retinoic acid in melanoma cells. Exp Cell Res 1995; 218: 263–70.
Sengupta S, Ray S, Chattopadhyay N, Biswas N, Chatterjee A. Effect of retinoic acid on integrin receptors of B16F10 melanoma cells. J Exp Clin Cancer Res 2000; 19: 81–7.
Rutz HP, Little JB. Modification of radiosensitivity and recovery from X ray damage in vitro by retinoic acid. Int J Radiat Oncol Biol Phys 1989; 16: 1285–8.
Niles RM, Loewy BP. Induction of protein kinase C in mouse melanoma cells by retinoic acid. Cancer Res 1989; 49: 4483–7.
Andersson E, Rosdahl I, Torma H, Vahlquist A. Ultraviolet irradiation depletes cellular retinol and alters the metabolism of retinoic acid in cultured human keratinocytes and melanocytes. Melanoma Res 1999; 9: 339–46.
Wood WR, Seftor EA, Lotan D, Nakajima M, Misiorowski RL, Seftor RE, et al. Retinoic acid inhibits human melanoma tumor cell invasion. Anticancer Res 1990; 10: 423–32.
Yongshan Y, DeBauche DM, Stanley WS. Epidermal growth factor receptor expression in a retinoic acid-treated human melanoma cell line. Cancer Genet Cytogenet 1990; 46: 261–9.
Dhingra K, Papadopoulos N, Lippman S, Lotan R, Legha SS. Phase II study of alpha-interferon and 13-cis-retinoic acid in metastatic melanoma. Invest New Drugs 1993; 11: 39–43.
Lee JH, Kishikawa M, Kumazoe M, Yamada K, Tachibana H. Vitamin A enhances antitumor effect of a green tea polyphenol on melanoma by upregulating the polyphenol sensing molecule 67-kDa laminin receptor. PLoS One 2010; 5: e11051.
Talwar HS, Griffiths CE, Fisher GJ, Russman A, Krach K, Benrazavi S, et al. Differential regulation of tyrosinase activity in skin of white and black individuals in vivo by topical retinoic acid. J Invest Dermatol 1993; 100: 800–5.
Siddikuzzaman, Grace VM. Anti-Metastatic Study of Liposome- Encapsulated All trans Retinoic Acid (ATRA) in B16F10 Melanoma Cells-Implanted C57BL/6 Mice. Cancer Invest 2014.
Yao J, Zhang L, Zhou J, Liu H, Zhang Q. Efficient simultaneous tumor targeting delivery of all-trans retinoid acid and Paclitaxel based on hyaluronic acid-based multifunctional nanocarrier. Mol Pharm 2013; 10: 1080–91.
Hsu SL, Lin YF, Chou CK. Transcriptional regulation of transferrin and albumin genes by retinoic acid in human hepatoma cell line Hep3B. Biochem J 1992; 283 (Pt 2): 611–5.
Hsu SL, Lin YF, Chou CK. Retinoic acid biphasically regulates the gene expression of hepatitis B virus surface antigen in human hepatoma Hep3B cells. J Biol Chem 1993; 268: 23093–7.
Tsao YP, Tsao LT, Hsu SL, Chen SL. Retinoic acid represses the gene expression of topoisomerase II in HEP3B cells. Cancer Lett 1994; 87: 73–7.
Hsu SL, Lin HM, Chou CK. Suppression of the tumorigenicity of human hepatoma hep3B cells by long-term retinoic acid treatment. Cancer Lett 1996; 99: 79–85.
Hsu SL, Wu WS, Tyan YS, Chou CK. Retinoic acid-induced apoptosis is prevented by serum albumin and enhanced by Lipiodol in human hepatoma Hep3B cells. Cancer Lett 1998; 129: 205–14.
Hsu SL, Chen MC, Chou YH, Hwang GY, Yin SC. Induction of p21(CIP1/Waf1) and activation of p34(cdc2) involved in retinoic acid-induced apoptosis in human hepatoma Hep3B cells. Exp Cell Res 1999; 248: 87–96.
Hsu SL, Cheng CC, Shi YR, Chiang CW. Proteolysis of integrin alpha5 and beta1 subunits involved in retinoic acid-induced apoptosis in human hepatoma Hep3B cells. Cancer Lett 2001; 167: 193–204.
Wei J, Ye C, Liu F, Wang W. All-trans retinoic acid and arsenic trioxide induce apoptosis and modulate intracellular concentrations of calcium in hepatocellular carcinoma cells. J Chemother 20141973947814Y0000000200.
Fu RD, Qiu CH, Chen HA, Zhang ZG, Lu MQ. Retinoic acid receptor-related receptor alpha (RORalpha) is a prognostic marker for hepatocellular carcinoma. Tumour Biol 2014; 35: 7603–10.
Hsu SL, Hsu JW, Liu MC, Chen LY, Chang CD. Retinoic acidmediated G1 arrest is associated with induction of p27(Kip1) and inhibition of cyclin-dependent kinase 3 in human lung squamous carcinoma CH27 cells. Exp Cell Res 2000; 258: 322–31.
Siddikuzzaman, Grace VM. Inhibition of metastatic lung cancer in C57BL/6 mice by liposome encapsulated all trans retinoic acid (ATRA). Int Immunopharmacol 2012; 14: 570–9.
Ramya D, Siddikuzzaman, Grace VM. Effect of all-trans retinoic acid (ATRA) on syndecan-1 expression and its chemoprotective effect in benzo(alpha)pyrene-induced lung cancer mice model. Immunopharmacol Immunotoxicol 2012; 34: 1020–7.
Arrieta O, Hernandez-Pedro N, Fernandez-Gonzalez-Aragon MC, Saavedra-Perez D, Campos-Parra AD, Rios-Trejo MA, et al. Retinoic acid reduces chemotherapy-induced neuropathy in an animal model and patients with lung cancer. Neurology 2011; 77: 987–95.
Lotan R. Different susceptibilities of human melanoma and breast carcinoma cell lines to retinoic acid-induced growth inhibition. Cancer Res 1979; 39: 1014–9.
Huber PR, Geyer E, Kung W, Matter A, Torhorst J, Eppenberger U. Retinoic acid-binding protein in human breast cancer and dysplasia. J Natl Cancer Inst 1978; 61: 1375–8.
Ong DE, Page DL, Chytil F. Retinoic acid binding protein: occurrence in human tumors. Science 1975; 190: 60–1.
Thulasiraman P, McAndrews DJ, Mohiudddin IQ. Curcumin restores sensitivity to retinoic acid in triple negative breast cancer cells. BMC Cancer 2014; 14: 724.
Marcato P, Dean CA, Liu RZ, Coyle KM, Bydoun M, Wallace M, et al. Aldehyde dehydrogenase 1A3 influences breast cancer progression via differential retinoic acid signaling. Mol Oncol 2014.
Berardi DE, Bessone MI, Motter A, Bal de Kier Joffe ED, Urtreger AJ, Todaro LB. Involvement of protein kinase C alpha and delta activities on the induction of the retinoic acid system in mammary cancer cells. Mol Carcinog 2014.
Arisi MF, Starker RA, Addya S, Huang Y, Fernandez SV. All transretinoic acid (ATRA) induces re-differentiation of early transformed breast epithelial cells. Int J Oncol 2014; 44: 1831–42.
Kamal AH, Han BS, Choi JS, Cho K, Kim SY, Kim WK, et al. Proteomic analysis of the effect of retinoic acids on the human breast cancer cell line MCF-7. Mol Biol Rep 2014; 41: 3499–507.
Alsafadi S, Even C, Falet C, Goubar A, Commo F, Scott V, et al. Retinoic acid receptor alpha amplifications and retinoic acid sensitivity in breast cancers. Clin Breast Cancer 2013; 13: 401–8.
Ombra MN, Di Santi A, Abbondanza C, Migliaccio A, Avvedimento EV, Perillo B. Retinoic acid impairs estrogen signaling in breast cancer cells by interfering with activation of LSD1 via PKA. Biochim Biophys Acta 2013; 1829: 480–6.
Chen Q, Ross AC. All-trans-retinoic acid and the glycolipid alphagalactosylceramide combined reduce breast tumor growth and lung metastasis in a 4T1 murine breast tumor model. Nutr Cancer 2012; 64: 1219–27.
Marchetti M, Russo L, Balducci D, Falanga A. All trans-retinoic acid modulates the procoagulant activity of human breast cancer cells. Thromb Res 2011; 128: 368–74.
Terao M, Fratelli M, Kurosaki M, Zanetti A, Guarnaccia V, Paroni G, et al. Induction of miR-21 by retinoic acid in estrogen receptorpositive breast carcinoma cells: biological correlates and molecular targets. J Biol Chem 2011; 286: 4027–42.
Ciolino HP, Dai Z, Nair V. Retinol inhibits aromatase activity and expression in vitro. J Nutr Biochem 2011; 22: 522–6.
Dutta A, Sen T, Chatterjee A. All-trans retinoic acid (ATRA) downregulates MMP-9 by modulating its regulatory molecules. Cell Adh Migr 2010; 4: 409–18.
Phipps SM, Love WK, White T, Andrews LG, Tollefsbol TO. Retinoid-induced histone deacetylation inhibits telomerase activity in estrogen receptor-negative breast cancer cells. Anticancer Res 2009; 29: 4959–64.
Hua S, Kittler R, White KP. Genomic antagonism between retinoic acid and estrogen signaling in breast cancer. Cell 2009; 137: 1259-71.
Chen H, Zhang H, Lee J, Liang X, Wu X, Zhu T, et al. HOXA5 acts directly downstream of retinoic acid receptor beta and contributes to retinoic acid-induced apoptosis and growth inhibition. Cancer Res 2007; 67: 8007–13.
Donato LJ, Suh JH, Noy N. Suppression of mammary carcinoma cell growth by retinoic acid: the cell cycle control gene Btg2 is a direct target for retinoic acid receptor signaling. Cancer Res 2007; 67: 609–15.
Pratt MA, Niu MY, Renart LI. Regulation of survivin by retinoic acid and its role in paclitaxel-mediated cytotoxicity in MCF-7 breast cancer cells. Apoptosis 2006; 11: 589–605.
Brandes D. Retinoic acid receptor and surface markers: models for the study of prostatic cancer cells. Prog Clin Biol Res 1981; 75B: 207–28.
Reese DH, Gordon B, Gratzner HG, Claflin AJ, Malinin TI, Block NL, et al. Effect of retinoic acid on the growth and morphology of a prostatic adenocarcinoma cell line cloned for the retinoid inducibility of alkaline phosphatase. Cancer Res 1983; 43: 5443–50.
Boyd D, Chisholm GD, Habib FK. Nuclear retinoic acid binding protein in human prostate adenomas. J Endocrinol 1985; 105: 157–62.
Boyd D, Beynon L, Chisholm GD, Habib FK. Characterization of the retinol and retinoic acid binding proteins in the human prostate. Cancer Res 1984; 44: 5532–7.
Jutley JK, Kelleher J, Whelan P, Mikel J. Cytosolic retinoic acid-binding protein in human prostatic dysplasia and neoplasia. Prostate 1987; 11: 127–32.
Halgunset J, Sunde A, Lundmo PI. Retinoic acid (RA): an inhibitor of 5 alpha-reductase in human prostatic cancer cells. J Steroid Biochem 1987; 28: 731–6.
Jutley JK, Reaney S, Kelleher J, Whelan P. Interactions of retinoic acid and androgens in human prostatic tissue. Prostate 1990; 16: 299–304.
Whelan P. Retinoic acid and prostatic cancer cell growth. Prog Clin Biol Res 1990; 357: 117–20.
Fong CJ, Sutkowski DM, Braun EJ, Bauer KD, Sherwood ER, Lee C, et al. Effect of retinoic acid on the proliferation and secretory activity of androgen-responsive prostatic carcinoma cells. J Urol 1993; 149: 1190–4.
Dahiya R, Boyle B, Park HD, Kurhanewicz J, Macdonald JM, Narayan P. 13-cis-retinoic acid-mediated growth inhibition of DU145 human prostate cancer cells. Biochem Mol Biol Int 1994;32:1-12.
Waghray A, Webber MM. Retinoic acid modulates extracellular urokinase-type plasminogen activator activity in DU145 human prostatic carcinoma cells. Clin Cancer Res 1995; 1: 747–53.
Webber MM, Waghray A. Urokinase-mediated extracellular matrix degradation by human prostatic carcinoma cells and its inhibition by retinoic acid. Clin Cancer Res 1995; 1: 755–61.
Gao M, Ossowski L, Ferrari AC. Activation of Rb and decline in androgen receptor protein precede retinoic acid-induced apoptosis in androgen-dependent LNCaP cells and their androgen-independent derivative. J Cell Physiol 1999; 179: 336–46.
Li MT, Richter F, Chang C, Irwin RJ, Huang H. Androgen and retinoic acid interaction in LNCaP cells, effects on cell proliferation and expression of retinoic acid receptors and epidermal growth factor receptor. BMC Cancer 2002;2:16.
Zhong C, Yang S, Huang J, Cohen MB, Roy-Burman P. Aberration in the expression of the retinoid receptor, RXRalpha, in prostate cancer. Cancer Biol Ther 2003; 2: 179–84.
Pandey KK, Batra SK. RXRalpha: a novel target for prostate cancer. Cancer Biol Ther 2003; 2: 185–6.
Chuang KH, Lee YF, Lin WJ, Chu CY, Altuwaijri S, Wan YJ, et al. 9-cis-retinoic acid inhibits androgen receptor activity through activation of retinoid X receptor. Mol Endocrinol 2005; 19: 1200–12.
Ameri A, Alidoosti A, Hosseini SY, Parvin M, Emranpour MH, Taslimi F, et al. Prognostic Value of Promoter Hypermethylation of Retinoic Acid Receptor Beta (RARB) and CDKN2 (p16/MTS1) in Prostate Cancer. Chin J Cancer Res 2011; 23: 306–11.
Gao T, He B, Pan Y, Li R, Xu Y, Chen L, et al. The association of retinoic acid receptor beta2(RARbeta2) methylation status and prostate cancer risk: a systematic review and meta-analysis. PLoS One 2013; 8: e62950.
Kim H, Lapointe J, Kaygusuz G, Ong DE, Li C, van de Rijn M, et al. The retinoic acid synthesis gene ALDH1a2 is a candidate tumor suppressor in prostate cancer. Cancer Res 2005; 65: 8118–24.
Huss WJ, Lai L, Barrios RJ, Hirschi KK, Greenberg NM. Retinoic acid slows progression and promotes apoptosis of spontaneous prostate cancer. Prostate 2004; 61: 142–52.
Kelsey L, Katoch P, Johnson KE, Batra SK, Mehta PP. Retinoids regulate the formation and degradation of gap junctions in androgen- responsive human prostate cancer cells. PLoS One 2012; 7: e32846.
Liu Z, Ren G, Shangguan C, Guo L, Dong Z, Li Y, et al. ATRA inhibits the proliferation of DU145 prostate cancer cells through reducing the methylation level of HOXB13 gene. PLoS One 2012; 7: e40943.
Lin E, Chen MC, Huang CY, Hsu SL, Huang WJ, Lin MS, et al. All-trans retinoic acid induces DU145 cell cycle arrest through Cdk5 activation. Cell Physiol Biochem 2014; 33: 1620–30.
Chen MC, Huang CY, Hsu SL, Lin E, Ku CT, Lin H, et al. Retinoic Acid Induces Apoptosis of Prostate Cancer DU145 Cells through Cdk5 Overactivation. Evid Based Complement Alternat Med 2012; 2012: 580736.
Culine S, Kramar A, Droz JP, Theodore C. Phase II study of alltrans retinoic acid administered intermittently for hormone refractory prostate cancer. J Urol 1999; 161: 173–5.
Trump DL, Smith DC, Stiff D, Adedoyin A, Day R, Bahnson RR, et al. A phase II trial of all-trans-retinoic acid in hormone-refractory prostate cancer: a clinical trial with detailed pharmacokinetic analysis. Cancer Chemother Pharmacol 1997; 39: 349–56.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Department of Life Sciences, National Chung Hsing University, No. 250, Kuokuang Rd., Taichung 402, Taiwan.
Department of Chest Medicine, Taichung Veterans General Hospital, No. 160, Taichung Harbor Rd., Sec. 3, Taichung 407, Taiwan.
These authors contributed equally to this work.
E-mail address: hlin@dragon.nchu.edu.tw (H. Lin), jonyin@gmail.com (T.-Y. Yang).
Open Access This article is distributed under terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided original author(s) and source are credited.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit https://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, MC., Hsu, SL., Lin, H. et al. Retinoic acid and cancer treatment. BioMed 4, 22 (2014). https://doi.org/10.7603/s40681-014-0022-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.7603/s40681-014-0022-1