Abstract
Initially neutral conditioned stimuli paired with food often acquire motivating properties, including serving as secondary reinforcers, enhancing instrumental responding in Pavlovian-instrumental transfer procedures, and potentiating food consumption under conditions of food satiation. Interestingly, cues associated with the cancellation of food and food cues may also potentiate food consumption (e.g., Galarce and Holland, 2009), despite their apparent negative correlations with food delivery. In three experiments with rats, we investigated conditions under which potentiation of feeding by such “interruption stimuIi” (ISs) develops, and some aspects of the content of that learning. Although in all three experiments ISs enhanced food consumption beyond control levels, they were found to act as conditioned inhibitors for anticipatory food cup entry (Experiment 1), to serve as conditioned punishers of instrumental responding (Experiment 2), and to suppress instrumental lever press responding in a Pavlovian instrumental transfer procedure (Experiment 3). Furthermore, when given concurrent choice between different foods, an IS enhanced consumption of the food whose interruption it had previously signaled, but when given a choice between performing two instrumental responses, the IS shifted rats’ choice away from the response that had previously yielded the food whose interruption had been signaled by IS (Experiment 3). Thus, the effects of an IS on appetitive responses were opposite to its effects on consummatory responding. Implications for our understanding of learned incentive motivation and the control of overeating are discussed.
Similar content being viewed by others
Introduction
Initially neutral stimuli paired with food often acquire motivating properties. For example, a food-paired conditioned stimulus (CS) typically enhances the rate of instrumental lever-pressing that earns that food reward (Pavlovian-instrumental transfer, or PIT; e.g., Holland, 2004, Holmes, Marchand, & Coutureau, 2010). Moreover, a CS paired with food while an animal is food-deprived can potentiate consumption of that food later when the animal is food-sated (Holland & Petrovich, 2005; Johnson, 2013; Weingarten, 1983). In addition to the direct evidence of learned incentive motivational function this latter observation provides, it may also provide a basis for understanding what may be a major contributor to the so-called “obesity epidemic” (e.g., Levitsky & Pacanowski, 2011), overconsumption of unneeded food in an environment rich in enticing food-related cues.
Interestingly, cues associated with the cancellation of food and food cues may also potentiate feeding. Galarce and Holland (2009) first trained rats with a 2-minute auditory CS during which a food unconditioned stimulus (US) was presented in a probabilistic fashion. Then, on some trials, that CS was terminated prematurely, whereupon another 10-second auditory cue (termed an “interruption stimulus” or IS) was presented. Later, when the rats were food-sated, that IS was found to potentiate consumption of the food reward, as did the CS. Subsequent experimentation (Galarce et al., 2010) replicated the basic observation of IS-potentiated feeding and showed that it shared several properties of CS-potentiated feeding, including specificity to the original food reward and dependence on function of the basolateral amygdala. However, Galarce and Holland (2009) also presented evidence suggesting that the rats had learned an inhibitory association between the IS and food: in the absence of food (and even early in a food consumption test), an IS presented alone suppressed food cup entry below baseline levels, and when combined with a CS, inhibited the food cup responses usually elicited by the CS. Furthermore, Holland and Hsu (2014) found that although both CSs and ISs potentiated feeding, only a CS enhanced the rate of instrumental responding in tests of PIT.
Here, we explored the apparent bivalence of IS learning in three experiments. In Experiment 1, we compared food cup entry and food consumption responses after training with an IS training procedure, traditional conditioned inhibition (CI) procedures in which an added stimulus accompanied non-reinforced presentations of the CS, and an unpaired (U) control procedure. In Experiment 2, we evaluated the ability of an IS to serve as a conditioned reinforcer or punisher by examining the effects of making delivery of an IS, a CS, or a control (U) cue contingent on operant lever-press responding. Finally, in Experiment 3, we examined the ability of IS and U cues to modulate choice among two available reinforcers and choice among two available instrumental responses that previously had produced those reinforcers. In all three experiments, the IS enhanced food consumption, but suppressed appetitive food-procuring responses.
Experiment 1
Experiment 1 was designed to compare the excitatory and inhibitory powers of an IS with those of more traditionally trained conditioned inhibitors. Previous studies of the potentiation of feeding by either CSs or ISs used unpaired discriminative stimuli as control stimuli. It is possible that any procedure that establishes conditioned inhibition to a stimulus might also endow that stimulus with the ability to enhance food consumption, for example through some sort of frustration-induced eating (Papini & Dudley, 1997).
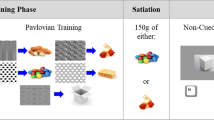
Figure 1 provides a schematic representation of the training procedures used in Experiment 1. All rats first received Pavlovian cue training to establish a CS, an unpaired discriminative control stimulus (U), and either an IS (Group IS) or a conditioned inhibitor (CI; Groups CI1 and CI2). In all groups, sucrose was delivered on a variable time (VT) 30-second schedule during 2-minute CS, but 10-second presentations of another (U) stimulus were never accompanied by sucrose. In Group IS, rats also received trials on which the reinforced CS was terminated prematurely and followed immediately by a 10-second IS cue. In Group CI1, IS trials were replaced with simultaneous non-reinforced compounds of the CS and the same stimulus that was used as IS in Group IS. Those compounds were matched in duration to the IS trials in Group IS. In Group CI2, the CI trials were identical to the IS trials in Group IS, except that no sucrose was delivered during either the CS or the subsequent CI stimuli. Thus, Group CI1 received procedures similar to a standard simultaneous conditioned inhibition procedure, and Group CI2 received procedures similar to a backward conditioned inhibition procedure (e.g., Holland, 1980). Then the rats received tests of these cues and various compound cues either while food-deprived and in the absence of the sucrose reinforcer in the liquid wells (cue test), or while food-sated and with sucrose continuously present in the liquid wells (cue-potentiated feeding test). The cue-potentiated feeding test permitted comparisons of the various cues’ abilities to modulate consumption of the sucrose reinforcer, and the cue test evaluated appetitive behavior (approach and entry to the liquid wells) unconfounded by the presence or consumption of that reinforcer.
Schematic representation of the cue-training procedures of Experiment 1. All groups of rats received identical reinforced CS (CS+) and unpaired (U) control stimulus trials. CS+ trials included sucrose deliveries on a variable time 30-second schedule (indicated by triangles) and were 120 seconds in duration. U trials were non-reinforced and 10 seconds in duration. Rats in Group IS also received interruption stimulus (IS) training trials, on which CS+ presentations were interrupted 30 seconds, 60 seconds, or 90 seconds after their onset, and followed by a 10-second presentation of the IS. In place of IS trials, rats in Group CI1 received simultaneous compound conditioned inhibition (CI) trials, which included presentations of CI1 and the CS, yoked in duration to the durations of IS trials in Group IS. No sucrose was presented at any time during CI1 trials. In Group CI2, IS trials were replaced by CI2 trials identical to IS trials except that no sucrose was presented. The tone served as CS+ for all rats, but the identities (white noise or clicker) of U and the IS, CI1, or CI2 were counterbalanced
Methods
Subjects
The subjects were 24 male Long-Evans rats (Charles River Laboratories, Raleigh, NC, USA), which weighed 275‒325 g on arrival to the laboratory vivarium. The rats were housed in individual cages in a vivarium room that was illuminated from 7:00 am to 7:00 pm. After a week of free access to food (2018 Rodent Diet, Harlan Teklad Laboratory, Madison, WI, USA) and water, the rats were given restricted food access so as to reach and maintain 85 % of their ad libitum weights. Behavioral training sessions were conducted near the beginning of the light-on period. The care and experimental treatment of the rats was approved by the Johns Hopkins University Animal Care and Use Committee.
Apparatus
There were eight training chambers (20.5 cm × 22.0 cm × 22.5 cm), with stainless steel front and back walls and clear acrylic side walls and tops. An illuminated, clear acrylic shallow liquid well, which could hold approximately 1.7 ml of liquid sucrose, was recessed into the center of the front wall. A small relay, used to signal liquid delivery in initial food-well training sessions, rested on top of the liquid well. Retractable response levers could be presented to the left or right of the liquid well; they were present only in portions of Experiments 2 and 3. A speaker for delivering a 78-db white noise cue, a piezoelectric device for presenting an intermittent (3 Hz) 79-db, 1900-hz tone, and a loud (78-db) relay clicker (4 hz) were mounted on the side wall of a sound-resistant shell that enclosed each chamber. Syringe pumps used to deliver liquids were mounted outside the sound-resistant shells; their operation was not detectable inside the chambers.
A photocell beam in the liquid well recess detected head entries and the time rats spent in the liquid well recess. A video camera mounted inside the sound-resistant shell was aimed at the area that included the liquid well recess to record the rat’s behaviors, and a second camera was located under the liquid well to record consummatory responses. To aid in video recording, a panel of infrared lights was placed on top of each experimental chamber. The camera images were digitized, recorded, and shown in real time on four video monitors. Each of these displayed images of four chambers or liquid wells.
Training procedures
Pavlovian cue training
The rats were first taught to approach and consume the sucrose reinforcer from the liquid wells. In each of two 64-minute sessions there were 16 0.1-ml deliveries of an 8 % sucrose solution, which served as the unconditioned stimulus (US). Each liquid delivery was accompanied by a single click provided by operation of the liquid well relay during these two sessions; the liquid well relay was disconnected for the remainder of the experiment. The intertrial intervals (ITIs) varied randomly between 2 and 8 minutes (mean = 4 minutes). Next, the rats were given six 60-minute Phase 1 training sessions designed to establish a Pavlovian association between a tone and sucrose. In each of these sessions, they received ten 2-minute presentations of the intermittent, 1900-hz tone CS. In nine of these CS trials, four USs were presented at random times on a variable time 30-second (VT 30 s) schedule. A single trial was selected as a CS ‘catch’ trial, which permitted assessing liquid well recess entries not confounded by the delivery of sucrose. On that trial, sucrose could not occur in the first 20 seconds (which served as the recording period), and the likelihood of sucrose delivery was increased in the remaining 100 seconds (VT 25 s) to produce the same overall density of reinforcement across all ten trials. The ITIs varied randomly between 3 and 12 minutes (mean = 6 minutes).
Next, the rats received fifteen 60-minute Phase 2 IS or CI training sessions. Each session included one CS catch trial and nine CS trials (as before), six unpaired cue trials (U), during which either the clicker or white noise stimulus (counterbalanced) was presented alone for 10 seconds, and six IS (Group IS, n = 8) or CI (Groups CI1 or CI2, ns = 8 each) trials. The ITIs varied randomly between 1.5 and 5.5 minutes (mean = 2.75 minutes). In Group IS, on IS trials the CS was presented and reinforced with sucrose in the same manner as on CS trials, except that 30 seconds, 60 seconds, or 90 seconds (two trials each) after its onset, the CS was terminated and a 10-second IS (either white noise or clicker, whichever did not serve as U) was presented. Regardless of the CS duration, the sucrose delivery density was maintained at VT 30 s during the CS. No sucrose presentations were permitted during or after the IS. In Group CI1, on CI1 trials the noise or clicker was presented in compound with the tone CS for 30 seconds, 60 seconds, or 90 seconds (two each), but no sucrose was presented. In Group CI2, CI trials were identical to IS trials in Group IS, except that no sucrose was presented at any time during those trials. Thus, Group CI1 provided training most like traditional feature-negative discrimination procedures, in which the excitor and inhibitor are presented simultaneously and not reinforced, whereas Group CI2 provided a backward CI procedure in which the CI occurred after the termination of non-reinforced presentations of the excitatory CS.
After cue training was completed, half of the rats in each group were given cue response tests, and half were given free access to food for 7 days in preparation for cue-potentiated feeding tests. After these tests were completed, the deprivation states were swapped for 7 days and the other type of test administered.
Cue-potentiated feeding test
After 7 days’ free access to their normal chow in their home cages, the sated rats in each group received a 20-minute potentiated feeding test on each of two successive days. One of these tests was designed to assess consumption induced by the CS, U, and either IS (Group IS) or CI (Groups CI1 and CI2) elements, and the other examined consumption during the CS, a CS+U compound, and either a CS+IS compound (Group IS) or a CS+CI compound (Groups CI1 and CI2). The inclusion of U trials provided a within-subject control for the abilities of the CS and IS cues to control eating. The CS+IS or CS+CI compound cues allowed a summation (Rescorla, 1969) assessment of the inhibitory (or excitatory) power of the IS or CI cues in the control of feeding, and the CS+U trials provided an unpaired control baseline for this summation test comparison. Half of the rats in each group received the elements test first and the other half received the compounds test first.
To satiate rats further on the sucrose US itself, immediately prior to each test session the rats were first given 10 minutes unlimited access to 15 ml of sucrose placed in cups attached to the floor of the experimental chamber, in front of the liquid well. After 10 minutes had elapsed, the rats were removed from the experimental chambers. The cups were removed and set aside for subsequent measurement of liquid consumption, and the liquid wells filled with 1.6 ml sucrose before the rats were replaced in the chambers for the 20-minute cue-potentiated feeding test.
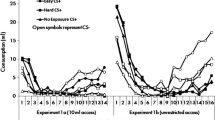
In the first 2-minute period of the cue-potentiated feeding test, no stimuli (other than sucrose) were presented. Over the next 18 minutes, the rats received five of each of the three types of cues and compounds, either CS, U, and IS (or CI) in the elements test, or CS, CS+U, and CS+IS (or CS+CI) in the compounds test. The trial order was counterbalanced for ordinal position and the immediately preceding cue type (as much as possible), and for each rat the cue sequence in the second test was the reverse of that in the first test. Each ITI was 1.2 minutes; they were held constant to minimize any differential effects of carryover from preceding trials. We chose 20-second test presentations to match the procedures used by Galarce and Holland (2009), Galarce et al. (2010), and Holland and Hsu (2014). Those studies showed that consumption was potentiated during 20-second CS or IS presentations compared to consumption during stimulus-free periods or U presentations.
The rats were tested in squads of four. During the test, the consumption behaviors of the rats were monitored (via the liquid well video cameras) by an experimenter who sampled each camera image once each second throughout the test session. When the liquid in a well was close to depletion, an additional 0.1 ml was delivered manually by signaling a computer program that activated the corresponding infusion pump and noted the timing and number of these deliveries, which served as a record of the pattern and quantity of sucrose consumed by each rat. To reduce the chance of experimenter bias, the experimenter was blind to whether a cue was being delivered or not, and to what cue each rat received on any trial. The measure of potentiated feeding was the difference between the rate of sucrose deliveries needed to maintain a constant sucrose well volume during stimulus presentations and the rate needed during stimulus-free periods. Following previous procedures (Galarce & Holland, 2009; Galarce et al., 2010; Holland & Hsu, 2014), we discarded data from the initial trials (one of each type) in each test session. First, despite home cage chow satiation and the availability of sucrose 10 minutes prior to the test, consumption continued at a high rate during the first few minutes of the test session. Second, initial presentations of the IS alone caused many of the rats to withdraw from the liquid well. This tendency might reflect conditioned inhibitory tendencies (Galarce & Holland, 2009) or the novelty of IS-alone presentations.
Cue response tests
Food-deprived rats received a 20-minute element or compound cue test on each of two successive days, in counterbalanced order. The cue tests were identical to the cue-potentiated feeding tests except that there was no sucrose prefeeding period nor sucrose delivery during the test session. Although these sessions were video recorded, only photocell measures of liquid well behaviors were analyzed.
Pavlovian response measure
The measure of conditioned liquid-well responding throughout the experiment was the percentage of time the liquid well photobeam was broken during various recording periods. In cue training, these periods included the 10-second pre-stimulus period on each trial, the 20-second sucrose-free period on CS+ catch trials, and the time during U, IS, CI+CS, or CI presentations. In the cue response tests, these periods included 20-second pre-stimulus and 20-second stimulus periods on each the four trial types. To reduce the effects of individual differences in tendencies to enter the liquid well, elevation scores were constructed by subtracting pre-stimulus responding from responding during the cues.
Data analysis
All response measures were subjected to repeated measures analyses of variance (ANOVAs) using the Greenhouse-Geisser correction to guard against any violations of sphericity. Group and counterbalancing variables (cue identities and test and subtest orders) were between-subject variables, and cue type and sessions (if relevant) were within-subject variables. Individual contrasts used the Tukey HSD procedure (adjusted for unequal ns as appropriate in Experiment 2).
Results
Pavlovian cue acquisition
Performance on CS catch trials increased slowly over the initial non-discriminative conditioning phase. On the last session, responding during the CS ranged from 36.1 % to 42.1 % across the three groups, and responding during the pre-CS periods ranged from 11.0 % to 15.1 %. One-way ANOVAs showed no effect of group (Fs < 1) for either measure.
The performance of the groups diverged after the various training procedures were introduced in Phase 2. Within a Group × Stimulus × three-session Block ANOVA of responding during Phase 2 training (Fig. 2), all main effects and interactions were significant (ps < .017) except the main effect of session blocks [F(4,84) = 1.75, p = .145]. Separate Group × Block ANOVAs for each stimulus were then conducted. For CS catch trials and U trials, only the effect of blocks was significant [Fs(21,84) > 56.87, ps < .001; other ps > .124]. By contrast, ANOVA of responding to the IS, CI1, and CI2 trials (in Groups IS, CI1, and CI2, respectively) showed significant effects of group [F(2,21) = 22.82, p < .001], session [F(4,84) = 28.86, p < .001], and their interaction [F(8,84) = 11.40, p < .001]. A post-hoc Tukey HSD test showed that responding to the IS was greater than responding to either CI1 or CI2 (ps < .001), which did not differ (p = .111). Post-hoc Tukey tests performed within the full Group × Stimulus × Blocks ANOVA showed that whereas responding to the IS was significantly (p < .001) lower than CS responding only on the last two three-session blocks, responding to the CI1 compound was lower than CS responding on each of the last three three-session blocks, and responding to the CI2 alone was lower than CS responding on all but the first block. A contrast over all session blocks combined showed that these differences were significantly greater in Group CI2 than in Group CI1, which in turn were greater than those in Group IS (ps < .001). Finally, a Group × Block ANOVA of pre-cue responding showed a significant effect of session block [F(4,84) = 5.04, p = .001] and a Group × Block interaction [F(8,84) = 2.23, p = .033]. However, pre-cue responding did not differ significantly across groups in the final block [F(2,21) = 2.80, p = .084].
Acquisition of liquid-well responding during training of the conditioned stimulus (CS), interruption stimulus (IS), conditioned inhibitors (CI) and unpaired (U) stimuli in Experiment 1. Entries are expressed as mean ± SEM percentage of time spent in the liquid well
The high levels of responding to IS during much of the acquisition phase are not surprising because IS was presented immediately after CS and US termination, and hence responding during IS likely reflected substantial carryover of liquid-well responding controlled by the CS and the US. Furthermore, it is notable that in Group IS, CS periods immediately prior to IS presentations often included US presentations, so the rats had their heads in the liquid wells nearly 100 % of the time (not shown). Thus, rats’ responding may have been more reduced by IS presentations than comparisons with the catch trial data might suggest.
Cue response test
Figure 3A shows the results of the cue response test, conducted while rats were food deprived and with no sucrose present in the liquid wells. A Group × Test Order × Subtest Order × Test Cue ANOVA found a significant effect of test cue [F(4, 48) = 532.58, p < .001] and a Group × Test Cue interaction [F(8,48) = 9.84, p < .001] but no other main effect or interaction was significant (ps > .124). Post-hoc individual comparisons among the test cues across groups used the Tukey HSD procedure. Whereas the CS evoked substantial liquid-well responding in all three groups, the IS and CIs did not. More importantly, when combined with the CS, the IS suppressed liquid-well responding (p < .001), but that suppression was significantly smaller than that produced by the CI in either Groups CI1 or CI2 (ps < .001). Although when compounded with the CS, the U cue also suppressed responding (ps < .001), that suppression was significantly smaller than that produced by the IS (p = .003) or CIs (ps < .001). Thus, the IS and both CI training procedures endowed those cues with inhibitory powers (as assessed by a summation test), compared to an unpaired control cue.
Performance in the liquid well entry tests and cue-potentiated feeding tests of Experiment 1. A: Mean ± SEM percentage of time spent in the liquid well during pre-cue intervals and during presentations of the conditioned stimulus (CS), interruption stimulus (IS; Group IS) or conditioned inhibitor or (CI; groups CI1 and CI2), and unpaired (U) control elemental stimuli, and of simultaneous compounds of CS and IS (Group IS) or CS and CI (Groups CI1 and CI2), and of CS and U. B: Mean ± SEM rates of 0.1-ml deliveries needed to maintain constant sucrose levels in the liquid wells during the periods identical to those described in panel A. In both panels, performance is pooled across the two test sessions (see text for procedures)
Pre-cue responding was 5.4 ± 0.7 %, 6.4 ± 1.3 %, and 6.7 ± 1.0 % in Groups IS, CI1, and CI2, respectively. A Group × Test Order × Subtest Order ANOVA of this responding showed no main effects or interactions involving group (ps > .508).
Half of the rats in each group received the cue response test after the cue-potentiated feeding test. Because those rats had free access to chow for a total of 9 days for that test, they gained considerable weight (below). They were deprived to 85 % of these new weights, and hence were heavier (421 ± 16 g) during the cue response test than the rats that received that test first (367± 13 g). Nevertheless, as noted previously, ANOVAs of cue responding showed no main effects or interactions involving test order.
Potentiation of feeding test
Rats gained considerable weight over the 7 days’ free access to chow that preceded the potentiated feeding test. The rats that received the cue-potentiated feeding test first weighed 471 ± 15 g at the time of the first test and the rats that received that test second weighed 480 ± 16 g.
Just prior to the cue-potentiated feeding tests, all rats were given access to 15 ml of sucrose in cups in the chambers for 10 minutes. Consumption in this pre-exposure period did not differ across groups, ranging from 8.9 ± 1.2 ml to 9.7 ± 1.6 ml (F < 1).
Figure 3B shows sucrose consumption during the cue-potentiated feeding tests themselves. A Group × Test Order × Subtest Order × Test cue ANOVA found a significant effect of test cue [F(4, 48) = 74.36, p < .001] and a Group × Test Cue interaction [F(8,48) = 8.86, p < .001]. There was a significant effect of test order (more consumption in the second test) [F(1,12) = 5.49, p = .037], but no other main effect or interaction was significant (ps > .101). Post-hoc individual comparisons among the test cues across groups used the Tukey HSD procedure. Relative to consumption during U, the CS enhanced consumption in all three groups (ps <.001). Although, again relative to U, the IS enhanced feeding in Group IS (p < .001), CI1 and CI2 did not do so in the two control groups (ps > .971), and consumption during IS was greater than that during CI1 or CI2 (ps < .008). Likewise, although both CI1 and CI2 both suppressed feeding in the presence of the CS (ps < .032), IS did not (p = .915), and consumption during the CS+IS compound was greater than consumption during the CS+CI1 or CS+CI2 compounds (ps < .001). Indeed, whereas the rats in Group IS consumed more during the CS+IS compound than during the CS+U compound (p = .025), the rats in Group CI1 consumed less during the CS+CI1 compound than during the CS+U compound (p = .002), and the rats in Group CI2 consumed comparable amounts during the CS+CI2 and CS+U compounds (p = .806). Thus, whereas IS was excitatory with respect to feeding, CI1, and to a lesser extent CI2, were inhibitory.
Pre-cue consumption during the tests was 5.9 ± 1.3, 5.4 ± 0.7, and 5.3 ± 1.2 deliveries/min in Groups IS, CI1, and CI2, respectively. A Group × Test Order × Subtest Order ANOVA of pre-cue consumption showed no significant main effects or interactions (ps > .117).
Discussion
In all three groups, a previously established CS for sucrose enhanced consumption of that sucrose reinforcer when rats were food-sated, as in previous studies. As observed by Galarce and Holland (2009) and Galarce et al. (2010), an IS that had previously accompanied the termination of the CS and cancellation of subsequent sucrose deliveries, also enhanced food consumption, compared to a control (U) stimulus. Furthermore, although the combination of IS and CS did not yield significant summation of their abilities to potentiate eating, the rats consumed numerically more during CS+IS compound presentations than during presentations of either CS or IS alone, so IS clearly did not inhibit the CS’s ability to potentiate eating. Thus the effect of IS on consumption was solely excitatory. By contrast, neither CI stimulus enhanced consumption relative to U, and both CIs suppressed consumption normally produced by a CS. Thus, the effects of the CIs on consumption were solely inhibitory. We can conclude then that CIs in general do not potentiate feeding.
At the same time, in a test of liquid well entry responding when the rats were food deprived and there was no sucrose in the wells, IS and both CIs inhibited liquid-well responding produced by presentation of the CS. Although the IS’s inhibitory powers were weaker than those of either of the other two CI procedures, the IS suppressed liquid-well responding to the CS more than did the control (U) cue. Thus, the IS was both excitatory with respect to sucrose consumption and inhibitory with respect to liquid well entry in the absence of sucrose.
It is worth noting that the three training procedures differed in many ways, perhaps obscuring the exact origins of these differences. For example, in Group IS, the tone CS was consistently reinforced, whereas in Groups CI1 and CI2, that stimulus was only partially reinforced. Nevertheless, the CS produced comparable consumption and liquid well entry in all three groups. Similarly, whereas rats in Group CI1 had considerable experience with simultaneous CS+CI compounds before the tests, neither Group CI2 nor Group IS had prior experience with simultaneous compounds. However, it is notable that whereas in Group IS consumption to the novel CS+IS compound was not lower than that during CS alone, in Group CI2 responding to that equally- ovel compound was as low as that to the familiar CS+CI compound in Group CI1.
Experiment 2
In Experiment 1, we found that although an IS was excitatory with respect to sucrose consumption, it was inhibitory with respect to a sucrose procurement response, liquid well entry. In Experiment 2 we examined whether another measure of cue value, conditioned reinforcement, would reveal an IS as a positively valued, reinforcing event, as indicated by its ability to enhance consumption, or as a negatively valued, punishing event, as implied by its ability to inhibit the performance of food procurement responses. Table 1 gives an outline of the procedures of Experiment 2. After training all rats with the procedure used in Group IS of Experiment 1, we established lever-pressing as an instrumental response yielding the same sucrose reinforcer. Next, the rats were divided into three groups, which received lever-press-contingent presentations of either CS, IS, or U in a test of conditioned reinforcement. CSs paired with primary reinforcers typically acquire the ability to reinforce instrumental responding, often thought to reflect the acquisition of positive incentive value by those CSs (e.g., Hendry, 1969; Mackintosh, 1974). Thus, rats in Group CS were expected to lever-press more in this test than rats in Group U, which earned a control stimulus after lever-press responses. If the IS enhanced food consumption because it acquired positive incentive value, rats in Group IS should also display more lever pressing than rats in Group U. By contrast, if the IS acquired negative incentive value, it would serve as a conditioned punisher (e.g., Dunham, 1971; Killcross et al, 1997; Seligman, 1966), that is, rats in Group IS would show less lever pressing than rats in Group U. Finally, all rats were given free access to food, and their sucrose consumption in the presences of CS, IS, and U was evaluated.
Subjects and apparatus
The subjects were 32 rats, obtained and maintained as in Experiment 1. The apparatus was that used in Experiment 1.
Training procedures
Pavlovian cue and instrumental response training
All rats were first given Pavlovian cue and IS training identical to that received by Group IS in Experiment 1. Next, all rats were trained to press each of the two levers to earn sucrose. Only one lever was available in each session. In the first lever press training session, each rat’s lever presses were reinforced on a fixed ratio 2 (FR 2) schedule until 100 lever presses were made, at which time the rat was removed from the chamber. All rats met this criterion within 60 minutes.
Instrumental lever-press training
The rats then received two daily 30-minute sessions of instrumental training for 6 days. Each day included one session with the left lever and one with the right lever, in counterbalanced order across days. Lever presses were reinforced with sucrose on a variable interval 30-second (VI 30 s) schedule. The instrumental response measure was the rate of lever pressing.
Finally, in preparation for the conditioned reinforcement/punishment tests, the rats were given a single IS training reminder session, identical to those received in Pavlovian cue training.
Conditioned reinforcement/punishment test
An instrumental choice test of conditioned reinforcement/punishment was then conducted. The primary purpose of this test was to determine whether IS training established appetitive or aversive properties to the IS. We also examined acquired properties of the CS to determine the ability of this test to reveal the acquisition of appetitive conditioned reinforcement expected with food-paired cues. In this 20-minute test, both the left and right levers were simultaneously present. Presses on the “cue lever” (arbitrarily selected left or right, counterbalanced) produced a 2-second presentation of the IS (Group IS, n = 10), CS (Group CS, n = 10), or U (Group U, n = 12) cue on a random ratio 5 (RR-5) schedule, and presses on the other, “no-cue lever”, had no consequence. Greater responding on the cue lever relative to responding on the no-cue lever was taken to mean that the cue had acquired appetitive reinforcement properties, and lower cue lever responding relative to no-cue lever responding indicated that the cue had acquired aversive, punishment properties. These differences were evaluated by contrast with the difference (if any) noted in Group U, which compared lever presses with no consequence and those that yielded presentations of the U control cue. No sucrose was delivered in this test.
We considered both the absolute response rates to each lever and a preference ratio [cue lever responding/(cue lever responding + no cue lever responding)]. The use of a preference ratio reduced variation produced by individual differences among the rats in the overall rate of lever-pressing.
At the conclusion of this test, the rats received another IS training reminder session, in preparation for subsequent food satiation and potentiated feeding testing.
Potentiated feeding test
After 7 days access to free chow in their home cages, the rats received a single potentiated feeding test identical to the element test administered in Experiment 1.
Results
Pavlovian cue acquisition
Acquisition during the CS+ and IS training phases proceeded as in Group IS of Experiment 1. Table 2 shows responding during each cue type in the last three-session block of training, in each of the three groups, which at this point had been treated identically. A Group × Period ANOVA showed only a main effect of period [F(3, 87) = 486.72, p < .001]. A post-hoc Tukey HSD showed that responding from each of the pre-cue, CS, IS, and U periods differed significantly (ps < .001) from responding during each of the other periods.
Instrumental response acquisition
All rats acquired instrumental lever-pressing rapidly. By the last session of VI 30-s training, the rats responded at comparable rates to the levers that would later be designated as cue lever (14.0 ± 1.6, 13.7 ± 1.5, and 13.8 ± 1.7 responses/minute in Groups IS, CS, and U, respectively) and no-cue lever (14.3 ± 1.7, 14.3 ± 1.7, and 13.6 ± 1.8 responses/minute). A Group × Cue Lever Identity (left or right) × Lever (subsequent assignment as cue lever or no-cue lever) showed no significant main effects or interactions (ps > .280).
Conditioned reinforcement test
The primary purpose of Experiment 2 was to determine whether an IS serves as a conditioned reinforcer or conditioned punisher, relative to an unpaired control stimulus. Figure 4 shows performance in the conditioned reinforcement test, in which pressing the cue lever was followed by presentations of either CS, IS, or U (in separate groups of rats) on an FR5 schedule, and pressing the other (no-cue) lever had no scheduled consequence. Figure 4A–E show lever press rates in each 5-minute block of the test, which was conducted in extinction (that is, no sucrose was available). Responding to both levers started at comparable high rates in all groups, but declined rapidly over the session. Cue response-contingent deliveries of the CS in Group CS (Fig. 4A) slowed the course of extinction relative to extinction of the no-cue response, whereas cue response-contingent deliveries of the IS in Group IS (Fig. 4B) hastened the course of extinction. Furthermore, the absolute value of the difference between cue and no-cue responding was greater in each of these groups than it was in Group U (Fig. 4C), in which the cue-lever produced the U, control, cue. Finally, preference ratios, cue lever responding/(cue lever + no-cue lever responding), were higher in Group CS than in Group U, but lower in Group IS than in Group U (Fig. 4F). This pattern of data suggests that the IS training regime established the CS as a conditioned reinforcer but the IS as a conditioned punisher.
Performance in the conditioned reinforcement tests of Experiment 2. After prior sucrose-reinforced training of responding to two levers, each rat was tested in a session in which a 2-second stimulus was presented after every five responses on the cue lever, while responses to the other, no-cue lever had no programmed consequences. Rats in the CS test group were tested with the conditioned stimulus, rats in the IS condition were tested with the interruption stimulus, and rats in the U condition were tested with the unpaired control stimulus as the response-contingent event. Panels A‒C show responding divided by test group, and panels D‒E show the same data arranged by lever. Panel F shows a lever preference ratio for the middle 50 % of the test session (see text for an explanation of why this test period was chosen). Ratios less than 0.5 indicate preference for the no-cue lever and ratios over 0.5 indicate preference for the cue lever
An initial ANOVA that included all the counterbalancing variables yielded no effects or interactions involving any of those variables (ps > .100), so they were dropped from the analysis. A Group (CS, IS or U) × Lever (cue or no-cue) × 5-minute Block ANOVA showed significant main effects of lever [F(1, 29) =5.83, p = .022] and block [F(3, 87) = 76.76, p < .001] as well as significant Group × Cue [F(2, 29) = 16.60, p < .001] and Group × Cue × Block [F(6, 87) = 4.63, p < .001] interactions. Trend analyses revealed a significant quadratic trend in the differences between responding on the cue and no-cue levers, overall, and in each individual group (ps < .002). These trends, showing maximum difference between the rates of responding on the cue and no-cue levers over the middle quarters of testing, reflect the likelihood that early in testing rats had not yet acquired the response➔cue contingencies, and late in testing responding had extinguished (e.g., Purgert et al., 2012). Most important, these trends differed significantly across each pair of groups (ps < .005). Comparable trend differences between the groups were also observed for responding on the cue levers alone (Fig. 4D, ps < .016), but for responding on the no-cue lever, the quadratic trends differed only between groups CS and IS (Fig. 4E, p = .018; other ps > .100). Post-hoc Group × Lever Tukey HSD contrasts (ps < .050) on responding summed over the entire tests showed that in Group IS, cue lever responding was significantly lower than no-cue lever responding, but the opposite was true in Group CS. These contrasts also showed that cue lever responding was significantly lower in Group IS than in either of Groups CS or U, and no-cue lever responding was significantly greater in Group IS than in Group CS. Finally, a one-way ANOVA of preference ratios across the groups was significant [F(2,29) = 42.24, p < .001]. Tukey HSD contrasts showed that each group differed significantly from each other group (ps < .002).
Potentiation of feeding test
After the completion of the conditioned reinforcement test, all rats were given free access to chow in their home cages, followed by a test designed to evaluate the potentiated feeding produced by the CS, IS, and U stimuli. The week of free access to chow prior to testing increased the rats’ weights from 356 ± 16 g, 350 ± 17 g, and 353 ± 18 g (deprived) in Groups CS, IS, and U, respectively, to 456 ± 12 g, 459 ± 17 g, and 458 ± 15 g. Prior to the test session, the rats were given 10-minute access to 15 ml of sucrose in cups placed in the experimental chambers. Consumption in this pretest was 9.6 ± 1.3, 9.6 ± 1.1. and 10.3 ± 1.3 in Groups CS, IS, and U, respectively. A one-way ANOVA showed no differences among the groups (F < 1).
Figure 5 shows sucrose consumption during CS, IS, U, and pre-cue periods during the test. Consumption was enhanced during presentations of either the CS or IS, compared to consumption during U or in empty (pre-cue) periods. Prior conditioned reinforcement testing condition (i.e., group membership) did not affect consumption; recall that these three groups received identical cue training. Thus, although the IS was demonstrated to be a conditioned punisher of instrumental behavior in the previous test (in Group IS), it enhanced sucrose consumption in this test in all three groups.
Consumption in the potentiated feeding tests of Experiment 2. Entries are the mean ± SEM rate of 0.1-ml deliveries needed to maintain constant sucrose levels in the sucrose wells during the pre-stimulus, conditioned stimulus (CS), interruption stimulus (IS), and unpaired control stimulus (U) periods, in each of the three groups that were defined by their prior conditioned reinforcement test procedures (shown in Fig. 4)
Initial ANOVAs that included all counterbalancing variables revealed no significant effects or interactions with those variables, so those variables were omitted from the final analysis. A Group × Cue ANOVA showed a significant effect of cue [F(2,58) = 92.19, p < .001] but no effect of group or Group × Cue interaction (ps > .935.) Tukey HSD contrasts showed that in each of the three groups, consumption during CS periods was greater than consumption during IS (ps < .010) or U (ps < .005), and consumption during IS periods was greater than during U periods (ps < .050). Finally, a one-way ANOVA of consumption during pre-CS periods showed no differences across the groups (F < 1, p = .936).
Discussion
We found that whereas a CS acted as a conditioned reinforcer for instrumental responding, an IS acted as a conditioned punisher. Rats that received response-contingent CS presentations pressed more on the cue lever than on the no-cue control lever, whereas those that received response-contingent IS presentations responded more on the control lever. Because in both cases the response-cue contingency was critical to the enhanced responding, these effects are not easily attributed to motivational or performance effects produced by simple presentation of the CS or IS, which would have affected both cue and no-cue lever pressing. Furthermore, both shifts in the distribution of responding across levers were greater than those found in rats that received U presentations for responding on the cue lever. Thus, it is reasonable to identify CS as a conditioned reinforcer and IS as a conditioned punisher. Nevertheless, in potentiated feeding tests, both CS and IS potentiated sucrose consumption relative to U’s effects. Thus, IS’s “value” was either negative or positive, depending on how it was assessed.
Experiment 3
Experiment 3 compared the influence of an IS on food consumption choice and on food procurement choice. It exploited Galarce et al.’s (2010) observation that the ability of an IS to potentiate consumption is specific to the reinforcer with which it was established. We first trained rats with two CSs, one paired with sucrose and one paired with maltodextrin. These two carbohydrates have similar caloric loads but, for rats, very different orosensory properties and are readily discriminated. We delivered them to separate liquid wells to permit assessment of the rats’ acquisition of selective CS-US associations. IS training was then conducted using one of the CSs and its reinforcer partner. Next, presses on one lever were reinforced with sucrose and presses on the other were reinforced with maltodextrin. Finally, after a week of free access to chow in their home cages, rats received presentations of IS or U superimposed on choice tests of consumption or of lever-pressing. In the lever-pressing (PIT) choice test, both levers were available, but neither reinforcer was presented. In the consumption (cue potentiated feeding) choice test, the levers were unavailable, but the two foods were continuously available in their respective liquid wells. Table 3 shows an outline of the procedures of Experiment 3.
Subjects and apparatus
The subjects were 16 rats, obtained and maintained as in Experiments 1 and 2.
The apparatus comprised eight chambers nearly identical to those used in Experiments 1 and 2. However, these chambers had recessed liquid wells on both ends of the chamber. Furthermore, the liquid wells were deeper, with much greater capacity (15 ml) than those in the chambers used in Experiments 1 and 2, and liquids were delivered via gravity-fed solenoid systems. Most important, these chambers did not permit us to record moment-by-moment liquid consumption, as in Experiments 1 and 2. Thus, we were limited to measuring consumption over an entire session in which only one type of stimulus was presented.
Because the two retractable levers were present on only one side of the chamber, there was asymmetry in travel time/effort to obtain the two reinforcers after lever presses, requiring extensive counterbalancing and complicating the data analysis, described later.
Training procedures
Pavlovian cue training
The rats first received two 60-minute sessions to train them to approach the liquid wells and consume the two food reinforcers. One of these sessions included 16 0.1-ml deliveries of 4 % (w/v) sucrose and one included 16 0.1-ml deliveries of 4 % (w/v) maltodextrin solution. The order of these sessions was counterbalanced among the rats. Each reinforcer was administered in a 4 % solution because pilot data from our laboratory indicated that at this concentration, they support similar consumption and Pavlovian learning rates in most Long-Evans rats. Next, the rats were given eight 60-minute sessions to establish two Pavlovian associations, CS1➔food1 and CS2➔food2. Because the two foods were delivered to different food wells, the rats’ approach to those wells provided a measure of the specificity of that learning. Each of the first four training sessions included ten presentations of one of the CS-reinforcer combinations: two sessions with CS1➔food1, and two sessions with CS2➔food2, in order 1221. Then, the rats received four 60-minute sessions in which trials with each of the reinforcers were intermixed within each session; in each of these sessions, there were five CS1➔food1 trials and five CS2➔food2 trials, randomly intermixed. The CSs were an intermittent (3 Hz) 82-dB, 1900-Hz tone and a steady 82-dB 4500-Hz tone and were each 2 minutes in duration. Four reinforcers were delivered at random times within each CS presentation. The identities of the CSs, food reinforcers, and their combinations were completely counterbalanced. The ITIs varied randomly between 3 and 12 minutes (mean = 6 minutes) in the sessions in these phases.
Next, all rats were given 15 sessions of IS training with a new auditory cue, either an 82-dB white noise or an 82-dB 3-Hz relay clicker (counterbalanced), specific to the CS1➔food1 combination. In each session, rats received one 2-minute CS1➔food1 trial, identical to those delivered previously. However, during four additional CS1➔food1 trials, the IS was presented, CS1 was terminated, and no further food1 deliveries were made. Five uninterrupted CS2➔food2 trials, and five presentations of a 10-second U stimulus (either clicker or noise, whichever did not serve as IS) were intermixed with these trials. The ITIs varied randomly between 2 and 8 minutes (mean = 4 minutes).
Instrumental lever-press training
The rats then received lever press training in which presses on the left and right levers earned different reinforcers (sucrose or maltodextrin, counterbalanced). For half of the rats that received each lever-reinforcer combination, that reinforcer was delivered to the liquid well between the two levers and for the other half, the reinforcer was delivered to the liquid well on the opposite wall. Only one lever was available in each training session. In the first lever press training session with each lever-reinforcer combination, each rat’s lever presses were reinforced on a fixed ratio 2 (FR 2) schedule until 100 lever presses were made, at which time the rat was removed from the chamber. All rats met this criterion within 60 minutes. The rats then received six 30-minute (VI-30) training sessions with each lever, with a random order of left- or right-lever sessions. After this training, the rats received a single reminder IS training session, identical to the ones received previously.
Satiation
After the completion of Pavlovian and instrumental training, the rats were given free access to lab chow in their home cages for a week in preparation for the choice tests. All rats then received two food consumption choice tests and two instrumental response choice tests while food-sated. The instrumental response choice tests were conducted under food satiation to equate the rats’ motivational state for the two types of tests. Half of the rats received the two food consumption choice tests first, and half received the two instrumental response choice tests first.
Food consumption choice tests
Each food consumption choice test began with access to each of the sucrose and maltodextrin reinforcers in cups attached to the floor of the experimental chamber, in front of one of the liquid wells (counterbalanced). A single cup, containing one of the foods (counterbalanced), was available when the rat was first placed in the chamber. After 3 minutes, the cup was replaced with a cup containing the other food for 6 minutes, after which the original cup was returned for an additional 3 minutes. The rats were then removed from the chambers, the cups removed and set aside for subsequent measurement of liquid consumption, and one liquid well was filled with 15 ml sucrose and the other with 15 ml maltodextrin. For half of the rats (Consistent condition), the foods were placed in the same liquid wells as had been used for deliveries of those reinforcers, and for the other rats (Inconsistent condition), they were placed in the opposite liquid wells. The rats were then placed back in the chambers for 11 minutes. There were no stimuli (other than the liquids in the wells) presented in the first 2 minutes. Then, the rats received nine 20-second presentations of either the IS or the U stimulus over the next 9 minutes. The ITIs were constant at 1 minute. Half of the rats tested in each condition (Consistent or Inconsistent) received IS presentations in the first test session and half received U presentations. The next day, the rats received a test session with the other cue. At the end of each test session, the rats were removed immediately and the amounts of liquid remaining in the liquid wells was measured with a syringe.
Instrumental response choice tests
Each instrumental response choice test began the same as the food consumption choice tests. Rats received pre-exposure successively to the two foods, and then were briefly removed from the chambers. However, unlike in the consumption tests, both levers, but neither food, were available in the chambers when the rats were replaced. Stimulus presentations were the same as in the consumption tests. Lever-press responding was assessed in extinction, that is, no reinforcers were delivered.
Data analysis
Because the large number of counterbalancing conditions prohibited complete counterbalancing across the 16 subjects, we collapsed across many of the counterbalancing conditions. ANOVAs of the acquisition data ignored future counterbalancing variables but included all current counterbalancing variables. For each test type (consumption or instrumental choice), initial ANOVAs included the primary independent variable of interest, test stimulus (IS or U), and the primary dependent variable, choice response (food1 vs. food 2 in the consumption choice test, or response1 vs. response 2 in the instrumental choice test). We combined these two within-subject variables with the various between-subject counterbalancing variables (two or three at a time) in a series of ANOVAs. If an ANOVA yielded effects or interactions for a variable significant at p < .10, that variable was retained; otherwise it was dropped from subsequent analyses. Our final analysis of the consumption test data was a Test Location of the food reinforcers (consistent or inconsistent with their location in training) × Test Stimulus × Choice Response ANOVA, and of the instrumental response test data was a Subtest Order × Test Stimulus × Choice Response ANOVA. In these final analyses, all other counterbalancing variables (CS identity, IS identity, response identity, test type order, pretest location of food1, first liquid presented in the pretest) were collapsed.
Results
Pavlovian cue acquisition
All rats learned to distribute most of their conditioned liquid-well responses to the appropriate liquid well, that is, to the food1 well during CS1 and to the food2 well during CS2. Figure 6 shows correct well and incorrect well responding over the final three-session block of IS training. Not surprisingly, rats directed more of their responses during IS to the food1 well, and divided their responding during the pre-CS periods and during the U cue between the two wells. For purposes of analysis, one well was arbitrarily assigned as the “correct” response during U for each rat. An IS Reinforcer Identity (sucrose or maltodextrin) × IS Identity (noise or clicker) × Response (correct or incorrect) × Stimulus (CS1, CS2, IS, or U) ANOVA showed no significant effects or interactions of reinforcer identity, so that variable was dropped from the analysis. An IS identity × Response × Stimulus ANOVA showed significant main effects of response [F(1, 14) = 359.70, p < .001] indicating more correct than incorrect responding, and stimulus, [F(3, 42) = 175.30, p < .001], reflecting less responding during the U control cue than during other cues. The Response × Stimulus interaction was also significant [F(3,42) = 134.36, p < .001], reflecting the comparable levels of correct and incorrect responses during the U control stimulus, but greater correct than incorrect responses during the other cues. Finally, IS identity interacted significantly with both response [F(1,14) = 5.24, p = .038] and stimulus [F(3,42) = 5.65, p = .002], reflecting more correct responding and less incorrect responding to IS when it was the clicker than when it was the noise. ANOVA of pre-cue responding (with correct and incorrect responses identified as those appropriate to the immediately-following stimulus) showed no differences between the two responses (F < 1, p = .577).
Performance in the last block of interruption stimulus training of Experiment 3. Entries are mean ± SEM percentage of time spent in the liquid wells. CS1 and CS2 were conditioned stimuli paired with delivery of foods 1 and 2, respectively. IS signaled interruption of CS1 and food1, as schematized in Fig. 1. U was an unpaired control stimulus. Correct responding to CS1 and IS signified responding to the food1 liquid well, and incorrect responding to those cues signified responding to the food2 liquid well. Correct and incorrect responses in the pre-cue periods were defined by the response that would be correct during the upcoming cue presentations. For each rat, food1 and food2 well responses were arbitrarily assigned as correct or incorrect responses during U presentations. The identities of CS1, CS2, food1 and food2 were counterbalanced, as were those of IS and U
Lever press acquisition
All rats acquired instrumental lever-pressing rapidly, responding at rates of 16.5 ± 1.5 and 15.2 ± 1.5 responses/minute to the levers that yielded food1 and food2, respectively, on the last session of VI 30-s training. An ANOVA that included the between-subjects counterbalancing variables of food1 identity (sucrose or maltedextrin), lever identity (left or right), and liquid well locus (between or opposite to the levers), and the repeated measure of response (food1- or food2-yielding lever presses) showed no significant main effects or interactions, ps > .215.
Satiation
Prior to either the PIT or consumption tests, the rats were given 7 days’ free access to chow in their home cages. Their weights increased from 359 ± 6 g to 447± 8 g over this period.
Pavlovian-instrumental transfer test
Figure 7A and B show the results of the PIT tests, conducted while the rats had free access to chow in their home cages, and with access to both levers simultaneously. When the IS was presented, the rats reallocated their responses away from the food1 lever to the food2 lever, whereas when U was presented, the rats’ allocation of responding was unaltered.
A Subtest Order (IS or U test first) × Period (IS, U, or pre-cue periods) × Response (food1 or food2 lever) ANOVA of lever press response rates (Fig. 7A) showed a significant main effect of response [F(1,14) = 12.91, p = .003] and a significant Period × Response interaction [F(2,28) = 10.96, p < .001]. Tukey HSD contrasts showed that the rate of food1 lever responding was significantly lower during IS than in the U (p = .029) or pre-cue (p = .018) periods, and was also lower than the rate of food2 lever responding during IS (p < .001). By contrast, neither response differed in rate between U and pre-CS periods (ps > .984). In addition, overall responding was greater in the first subtest than in the second subtest [F(2,28) = 11.26, p < .001], but the Subtest Order × Cue × Response interaction was not significant (F < 1, p = .381).
Performance in the Pavlovian-instrumental transfer and food consumption choice tests of Experiment 3. A: Mean ± SEM lever-press rates during the pre-stimulus, interruption stimulus (IS) and unpaired control stimulus (U) periods in the lever response choice tests. The set of bars labeled food1 refers to responding to the lever previously associated with food1, with which the IS was trained. The bars labeled food2 refer to responding to the lever previously associated with food2. B: Preference ratio for responding on the food1 lever during IS, U and pre-cue periods (food1 lever presses/total responses). Ratios less than 0.5 indicate preference for the food2 lever and ratios over 0.5 indicate preference for the food1 lever. C: Total consumption of each of the two foods (sucrose and maltodextrin) during the test sessions that included the IS or U stimulus. The IS had been associated with the cancellation of food1 and its signal. D: Preference ratio for consuming food1 during the IS and U test sessions. Ratios less than 0.5 indicate preference for food2 and ratios greater than 0.5 indicate preference for food1
A comparable analysis that excluded the pre-CS periods was also performed, to permit more direct comparisons with the results of the consumption tests (next section). That Subtest Order × Cue (IS or U) × Response ANOVA showed a significant main effect of response [F(1,14) = 14.07, p = .002] and a significant Cue × Response interaction [F(1,14) = 10.63, p = .006]. Tukey HSD contrasts again showed that the rate of food1 lever responding was significantly lower during IS than during U (p = .049), and was also lower than the rate of food2 lever responding during IS (p = .004). In addition, overall responding was greater in the first subtest than in the second subtest [F(1,14) = 22.72, p < .001], but the Subtest Order × Cue × Response interaction was not significant [F < 1, p = .361].
Finally, a Subtest Order × Cue ANOVA of the food1 responding/(food1 responding + food2 responding) preference ratios (Fig. 7B) showed only a significant main effect of cue [F(1,14) = 10.26, p = .006, other ps > .353].
Conditioned potentiation of feeding test
During the test that included IS presentations, rats consumed more of food1 (with which the IS had been trained) than of food2, but consumption of the two foods was equal during the test that included U (Fig. 7C). Preference ratios of the form food1/(food1 + food2) were substantially higher in the IS test than in the U test (Fig. 7D). A Reinforcer Location (consistent or inconsistent with training location) × Cue × Food ANOVA of the amounts of each food consumed showed significant effects of cue, F(1, 14) = 12.91, p = .003, food, F(1,14) = 19.24, p < .001, and their interaction F(1, 14) = 12.40, p = .004. The effect of reinforcer location (more consumption if the locations of the two food reinforcers in test were consistent with their locations in training) was marginally significant F(1,14) = 3.69, p = .075, but none of that variable’s interactions approached significance, ps > .188. Tukey HSD tests showed that consumption of food1 during the IS test was significantly greater (ps < .001) than consumption of food2 in that test or of either food reinforcer in the U test. Likewise, a Reinforcer Location × Cue ANOVA of the preference ratios yielded a significant main effect of cue, F(1,14) = 12.58, p = .003, but no effect of reinforcer location or its interaction with cue (ps > .335).
Discussion
Presentations of an IS selectively enhanced consumption of the food whose cancellation it signaled, even when the food reinforcers were placed in the food wells opposite to where they had been previously experienced. Thus, the IS-potentiated feeding effect was both robust and food-specific, as noted previously by Galarce et al. (2010). By contrast, the IS selectively suppressed pressing of the lever that previously had earned that same reinforcer. Thus, the IS had opposite effects on food consumption and food procurement responses, extending the results of Experiments 1 and 2 to another measure of IS’s influence on food procurement behavior, selective PIT. Furthermore, unlike in Experiments 1 and 2, the assessments of consumption and lever pressing were conducted under similar conditions of stimulus presentation and food satiation, further extending our knowledge of the conditions under which IS’s express opposite influence on food consumption and procurement.
Several aspects of the PIT test warrant discussion. First, the IS appeared to show negative transfer to the lever-press response that was previously reinforced with food1 (with which the IS was trained), but positive transfer to the lever-press responses that had been reinforced with food2. We interpret the enhanced food2-lever responding as secondary to the suppression of food1-lever responding: both levers were simultaneously available so reductions in one response rate freed the other response from competition. Although it is possible that PIT may be greater when cues are superimposed on baselines of responding for somewhat different reinforcers, it is more commonly reported that suppression occurs when the CS and instrumental response reinforcers are mismatched, and enhancement when they match (e.g., Delamater & Holland, 2008). PIT Tests in which only a single lever was available might have been informative in this regard. Regardless, it is quite clear that, unlike with CSs, there was no positive transfer between an IS and an instrumental response based on the same reinforcer.
This lack of positive PIT with an IS is consistent with Holland and Hsu’s (2014) failure to find IS-produced PIT in single-reinforcer IS and instrumental response training, extending those authors’ observations to testing under conditions of food satiation (rather than under food deprivation) and when a single cue type (rather than all types) were presented in each test session. However, one might ask why the negative transfer observed here did not occur in Holland and Hsu’s (2014) study. It is possible that providing an alternative response in the present experiment, or other procedural differences, yielded a more sensitive measure of PIT, or that the mechanisms of one- and two-reinforcer PIT differ. For example, some researchers (e.g., Holland, 2004) have suggested that whereas single-reinforcer PIT reflects an incentive motivation process by which the CS energizes instrumental responding, multi-reinforcer PIT involves a signaling process, whereby the CS evokes a reinforcer representation that provides additional cues for performing the response that leads to that reinforcer. By inhibiting the food1 representation, the IS might counteract such cues provided by the context, levers, and so forth. Furthermore, some investigators have claimed that these two functions are mediated by different brain systems, with the general function requiring integrity of the amygdala central nucleus but the specific function requiring function of the basolateral amygdala (e.g., Corbit & Balleine, 2005).
General discussion
In three experiments, an IS that was associated with the termination of a CS and the cancellation of deliveries of a liquid sucrose reinforcer enhanced consumption of that sucrose, but suppressed various aspects of appetitive behavior preparatory to receiving sucrose. In Experiment 1, the IS was shown to be a conditioned inhibitor of liquid-well responding in a summation test; in Experiment 2, the IS was found to serve as a conditioned punisher of lever-press responding; and in Experiment 3 the IS suppressed ongoing performance of instrumental lever-pressing for the reinforcer with which the IS had been established. Thus, the effects of the IS on consummatory behavior were opposite to its effects on appetitive behavior. These results join others in differentiating between properties of appetitive and consummatory behavior (Craig, 1918), and in showing that “motivation to eat” and “motivation to procure” food can be independent (e.g., Berridge, 2004; Ikemoto & Panksepp, 1996). For example, investigations of habit learning describe conditions under which rats readily perform instrumental responses to obtain food reinforcers, which because of post-training pairings with toxins, are actively rejected (e.g., Yin, Knowlton, & Balleine, 2004). Conversely, dieters may work assiduously to avoid high-energy foods but gorge on them when confronted with the foods themselves.
Selection of “excitatory” over “inhibitory” aspects of the IS’s control over behavior was not simply tied to deprivation state. In Experiment 3, rats were under the same food-satiation conditions when IS was found to potentiate eating as when it was found to suppress lever pressing for that reinforcer. Although the presence/absence of food may have served such a disambiguating or occasion-setting role in these experiments, it is unlikely to have been the only determinant. Holland and Hsu (2014) found no evidence for positive PIT with an IS when it was assessed on baseline, that is, while the reinforcers were still available on the VI lever-pressing schedule.
The observation in Experiment 3 that the IS’s effects on consummatory behavior were specific to the food reinforcer whose cancellation it signaled suggests that ISs, like CSs, code detailed representations of the reinforcer. This coding may reflect the establishment of direct, backward associations with the US on IS training trials, or might be mediated by associations with the CS whose termination IS accompanied on those trials. It is possible that the ability of an IS to potentiate consumption is mediated by excitatory associations between IS and CS, at the same time that IS is linked to the absence of the food US via inhibitory associations with that US (e.g., Holland & Rescorla, 1975). Such mediation by IS-CS associations would be consistent with observations that both IS- and CS-potentiation of feeding share US-specificity and dependence on function of the basolateral amygdala. It would be interesting to determine the effects of treatments that extinguished those associations on IS’s ability to potentiate eating (Rescorla, 1980). Regardless of the route by which such a reinforcer representation is evoked by the IS, it apparently mediates different aspects of behavior in different ways. Whereas the IS selectively enhanced consumption of the food presented on IS trials, it selectively suppressed instrumental responses that ordinarily earned that food.
Although the IS training procedure could be construed as a conditioned inhibition procedure, and indeed in Experiment 1, the IS was shown to be a conditioned inhibitor of liquid cup behavior, conditioned inhibitors established with other procedures, including more typical simultaneous or backward feature negative discrimination procedures, did not potentiate eating in Experiment 1. Thus, the IS did not appear to potentiate eating solely because it was a conditioned inhibitor. Holland and Hsu (2014) considered a related possibility, that an IS enhances eating because it generates an aversive state, such as stress or frustration. Stress-induced and frustration-induced eating are well documented (e.g., Levine & Morley, 1981; Polivy & Herman, 1999). Within this view, there is no need to describe IS’s properties as bivalent: it is simply an inhibitory cue, which induces an aversive state. That state in turn punishes and suppresses lever pressing, but potentiates eating. However, Holland and Hsu (2014) found no evidence that IS-potentiated eating was affected by lesions of amygdala central nucleus, which reduce many frustration-induced enhancements of behavior (e.g., Henke, 1973; Henke & Maxwell, 1973; Kemble & Beckman, 1970), suppress stress responses, and generally impair the acquisition or performance of negative affective responses in learning (e.g., Fanselow & Poulos, 2005). Furthermore, cues with aversive properties established by pairing with shock suppress, rather than enhance, food consumption, even when obvious response competition (e.g., from freezing) is eliminated (Petrovich, Ross, Mody, Holland, & Gallagher, 2009).
The question of the conditions necessary for the acquisition of an IS’s apparently bivalent properties remains. The IS training procedure used here includes a relation between the IS and both a reduction in US frequency and termination of the CS, and backward relations with the presence of both US and CS. Galarce and Holland (2009) concluded that the IS’s relation to CS termination might be more important to its ability to enhance consumption than its relation to US frequency reduction. In an experiment modeled after that of Kamin (1956), which was intended to parcel out the effects of warning signal termination and shock frequency reduction on avoidance learning, Galarce and Holland (2009) attempted to isolate the roles of CS termination and US frequency reduction in the acquisition of IS-induced eating. In that experiment, rats in Group CSUS received training similar to the IS training given in the present experiments. Rats in Group CS received IS training trials in which the IS signaled CS termination in the same manner as in Group CSUS, but the number of USs presented on IS training trials was held constant regardless of CS duration. For the rats in Group US, IS presentations canceled all future USs on that trial, as in Group CSUS, but the CS was not terminated. Finally, in a control group the “IS” was presented apart from CS trials, as in the unpaired (U) control procedure used in the present experiments. In subsequent consumption tests, although the IS enhanced eating in all but the control group, the potentiation in Group US was significantly smaller than that in Group CSUS and Group CS, which did not differ. Thus, not only did IS’s relation with food frequency reduction alone establish only marginally significant tendencies to enhance eating, but adding that relation to the IS-CS termination relation in Group CSUS did not enhance IS’s potentiation beyond the level observed with the IS-CS termination relation alone in Group CS.
However, the results of Experiment 1 of the present series show that arranging a relation between a stimulus and CS termination is not sufficient to establish that stimulus as an IS. The CI2 cue in Group CI2 was paired with CS termination exactly as the IS in Group IS. Notably, CI2 was also associated with an even greater reduction in US frequency (to zero) during the CS than in Group IS. Nevertheless, the CI2 cue did not enhance eating. Thus, neither CS termination nor food frequency reduction alone, nor both in combination, was sufficient condition for the acquisition of the ability to potentiate feeding. The major difference between IS and CI2 training trials in Experiment 1 was the presentation of sucrose during the CSs on IS trials but not on CI2 training trials, suggesting that the relation between IS and the US itself, rather than US frequency reduction, is a condition critical for the IS’s ability to enhance eating, together with its relation to CS termination. Alternately, consistent with the possibility that IS-CS associations might mediate IS’s ability to potentiate eating, it might be argued that rats distinguish between reinforced and non-reinforced CS presentations, and IS was paired with a reinforced CS only in Group IS.
Apart from such mechanistic concerns, it is worth considering these results at a more descriptive level. Although the results of these experiments seem paradoxical within a simple excitation-inhibition account of the IS’s motivational significance, the casual notion that an IS signals impending food scarcity is consistent with both its potentiation of consumption and its suppression of procurement responses. Galarce and Holland (2009) suggested that IS-potentiated consumption reflects an adaptive response to the anticipation of future scarcity of a resource, whereas if that resource is already absent, approaching (Experiment 1) or working to earn (Experiment 2) further signals of its absence seems maladaptive. Similarly, in Experiment 3, when two preferred foods are present and a signal for the future scarcity of one of them is presented, the adaptive choice is to first consume the one whose scarcity is signaled, maximizing consumption while it is still available. Indeed, the presence of that food despite signals to the contrary might be viewed as an unexpected delight, and hence of increased value. By contrast, when the foods themselves are absent but opportunities to procure them are available, pursuing the food that is known to be scarce seems considerably less adaptive than pursuing a food thought to be still available. Importantly, the local mechanisms by which an IS acquires orexigenic powers need not directly represent the ultimate adaptive consequences of those mechanisms. For example, as Galarce and Holland (2009) noted, termination of food cues (including distal cues of food itself) may be a reliable token of food scarcity that is more readily learned about than a more molar representation of food frequency or probability. More generally, the IS need not represent “food scarcity” itself to enhance eating, but may do so as a result of its participation in a set of excitatory and inhibitory associations with food cues and food itself, as suggested earlier.
Finally, these effects may be relevant to eating and overeating in humans, especially those who have experienced “food insecurity” (uncertainty and interruptions in their food supply), and those individuals who have been described as “restrained eaters” (Herman & Polivy, 1980). Urbszat, Herman, and Polivy (2002) found that the threat of future unavailability of a food item can induce increased consumption of that item. Participants in a dieting study were warned that later they would be forbidden to eat certain forbidden foods, such as pizza, chips, and cookies. Before the restrictions were put in place, however, participants were allowed to consume unlimited quantities of one of those items, ostensibly to rate its tastiness. Restrained eaters consumed more of that item than restrained eaters who were not faced with the prospect of future prohibition against consuming that item, and more than unrestrained eaters who anticipated the same restrictions. Thus, a range of evidence suggests that both food abundance and food scarcity, and cues associated with those conditions, may interact in many ways to alter the pursuit and consumption of food.
References
Berridge, K. C. (2004). Motivation concepts in behavioral neuroscience. Physiology & Behavior, 81, 179–209.
Corbit, L. H., & Balleine, B. W. (2005). Double dissociation of basolateral and central amygdala lesions on the general and outcome-specific forms of Pavlovian-instrumental transfer. Journal of Neuroscience, 25, 962–970.
Craig, W. (1918). Appetites and aversions as constituents of instincts. Biological Bulletin, 34, 91–107.
Delamater, A. R., & Holland, P. C. (2008). The influence of CS-US interval on several different indices of learning in appetitive conditioning. Journal of Experimental Psychology: Animal Behavior Processes, 34, 202–222.
Dunham, D. J. (1971). Punishment: Method and theory. Psychological Review, 78, 58–70.
Fanselow, M. S., & Poulos, A. M. (2005). The neuroscience of mammalian associative learning. Annual Review of Psychology, 56, 207–234.
Galarce, E. M., & Holland, P. C. (2009). Effects of cues associated with meal interruption on feeding behavior. Appetite, 52, 693–702.
Galarce, E. M., McDannald, M. A., & Holland, P. C. (2010). The basolateral amygdala mediates the effects of cues associated with meal interruption on feeding behavior. Brain Research, 1350, 112–122.
Hendry, D. P. (1969). Conditioned reinforcement (p. 1969). Homewood, Illinois: Dorsey Press.
Henke, P. G. (1973). Effects of reinforcement omission on rats with lesions in the amygdala. Journal of Comparative and Physiological Psychology, 84, 187–193.
Henke, P. G., & Maxwell, D. (1973). Lesions in the amygdala and the frustration effect. Physiology and Behavior, 10, 647–650.
Herman, C. P., & Polivy, J. (1980). Restrained eating. In A. Stunkard (Ed.), Obesity (pp. 208–225). Philadelphia: W. B. Saunders.
Holland, P. C. (1980). Second order conditioning with and without unconditioned stimulus presentation. Journal of Experimental Psychology: Animal Behavior Processes, 6, 238–250.
Holland, P. C. (2004). Relations between Pavlovian-instrumental transfer and reinforcer devaluation. Journal of Experimental Psychology: Animal Behavior Processes, 30, 104–117.
Holland, P. C., & Hsu, M. (2014). Role of amygdala central nucleus in the potentiation of consuming and instrumental lever-pressing for sucrose by cues for the presentation or interruption of sucrose delivery in rats. Behavioral Neuroscience, 128, 71–82.
Holland, P. C., & Petrovich, G. D. (2005). A neural systems analysis of the potentiation of feeding by conditioned stimuli. Physiology and Behavior, 86, 747–761.
Holland, P. C., & Rescorla, R. A. (1975). Second order conditioning with food unconditioned stimulus. Journal of Comparative and Physiological Psychology, 88, 459–467.
Holmes, N. M., Marchand, A. R., & Coutureau, E. (2010). Pavlovian to instrumental transfer: A neurobehavioural perspective. Neuroscience and Biobehavioral Reviews, 34, 1277–1295.
Ikemoto, S., & Panksepp, J. (1996). Dissociations between appetitive and consummatory responses by pharmacological manipulation s of reward-relevant brain regions. Behavioral Neuroscience, 110, 331–345.
Johnson, A. W. (2013). Eating beyond metabolic need: How environmental cues influence feeding behavior. Trends in Neurosciences, 36, 101–109.
Kamin, L. J. (1956). The effects of termination of the CS and avoidance of the US on avoidance learning. Journal of Comparative and Physiological Psychology, 49, 420–424.
Kemble, E. D., & Beckman, G. J. (1970). Runway performance of rats following amygdaloid lesions. Physiology & Behavior, 5, 45–47.
Killcross, A. S., Everitt, B. J., & Robbins, T. W. (1997). Symmetrical effects of amphetamine and alphaflupenthixol on conditioned punishment and conditioned reinforcement: Contrasts with midazolam. Psychopharmacology, 129, 141–152.
Levine, A. S., & Morley, J. E. (1981). Stress-induced eating in rats. American Journal of Physiology, 241, R72–R76.
Levitsky, D. A., & Pacanowski, C. R. (2011). Free will and the obesity epidemic. Public Health and Nutrition, 15, 126–141.
Mackintosh, N. J. (1974). The psychology of animal learning. New York: Academic Press.
Papini, M. R., & Dudley, R. T. (1997). Consequences of surprising reward omission. Review of General Psychology, 1, 175–197.
Petrovich, G. D., Ross, C. A., Mody, P., Holland, P. C., & Gallagher, M. (2009). Central, but not basolateral amygdala, is critical for control of feeding by aversive learned cues. Journal of Neuroscience, 29, 15205–15212.
Polivy, J., & Herman, C. P. (1999). Distress and eating: Why do dieters overeat? International Journal of Eating Disorders, 26, 153–164.
Purgert, R. J., Wheeler, D. S., McDannald, M. A., & Holland, P. C. (2012). Role of amygdala central nucleus in aversive learning produced by shock or by unexpected omission of food. Journal of Neuroscience, 32, 2461–2472.
Rescorla, R. A. (1969). Pavlovian conditioned inhibition. Psychological Bulletin, 72, 77–94.
Rescorla, R. A. (1980). Pavlovian second-order conditioning: Studies in associative learning. Hillsdale, N. J.: Erlbaum.
Seligman, M. E. P. (1966). CS redundancy and secondary punishment. Journal of Experimental Psychology, 72, 546–550.
Urbszat, D., Herman, C. P., & Polivy, J. (2002). Eat, drink, and be merry, for tomorrow we diet: Effects of anticipated deprivation on food intake in restrained and unrestrained eaters. Journal of Abnormal Psychology, 111, 396–401.
Weingarten, H. P. (1983). Conditioned cues elicit feeding in sated rats: A role for learning in meal initiation. Science, 220, 431–433.
Yin, H. H., Knowlton, B. J., & Balleine, B. W. (2004). Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. European Journal of Neuroscience, 19, 181–189.
Acknowledgments
These data were presented at the 25th Congress of the Spanish Society for Comparative Psychology, September 2013. I thank Ezequiel Galarce for his helpful comments on this research, and Connor Galvin, Caitlin Hepps-Keeney, and Melanie Hsu for their technical assistance. The research was supported in part by NIH grant MH53667.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Holland, P.C. Stimuli associated with the cancellation of food and its cues enhance eating but display negative incentive value. Learn Behav 42, 365–382 (2014). https://doi.org/10.3758/s13420-014-0154-x
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13420-014-0154-x