Abstract
The potentiation of neural activity in lateral prefrontal regions via transcranial direct current stimulation (tDCS) can reduce patterns of biased attention for threat and may facilitate intentional emotion regulation. The current study sought to determine whether left dorsolateral prefrontal cortex tDCS, in combination with intentional down-regulation of emotional responses would reduce negative appraisals of aversive content during emotional regulation (assessed during online tDCS), reduce patterns of biased attention and attention bias variability (assessed offline), and attenuate spontaneous (uninstructed) emotional reactivity to negative content (assessed offline) above tDCS or intentional down-regulation of emotions in isolation. Healthy participants (n = 116) were allocated to one of four experimental conditions involving either active or sham tDCS, combined with an either a down-regulate or maintain emotion regulation task. Attention bias/bias variability was assessed with an attentional probe task, and emotional reactivity was assessed in a negative video viewing task. tDCS did not affect the appraisals of negative stimuli during emotion regulation, and there were no effects on attention bias/bias variability. However, tDCS did attenuate emotional reactivity. Those receiving active stimulation showed smaller elevations in negative mood in response to viewing aversive video content compared with sham. The present findings are consistent with the potential of left frontal tDCS to attenuate negative emotional reactions to aversive content but provide no support for tDCS enhancement of emotion regulation, nor its impact on attention bias or attention bias variability.
Similar content being viewed by others
The accumulation of extensive neuroimaging research and resulting neurocognitive models of psychopathology has informed potential targets for noninvasive neurostimulation techniques (Bishop, 2007). These techniques, such as transcranial direct current stimulation (tDCS), seek to augment neural activity in regions implicated in the maintenance of emotional disorders. As a result, a considerable body of literature has accumulated in recent years showing the potential promise of tDCS for the treatment of depressive disorders (Brunoni et al., 2016). Albeit a number of studies have shown limited (Loo et al., 2012) or no benefit (Palm et al., 2012). Although considerably less advanced than research on depression, a number of studies also have shown potential promise for tDCS as a treatment for anxiety disorders (Vicario, Salehinejad, Felmingham, Martino, & Nitsche, 2019). In addition to clinical applications, neurostimulation techniques, such as tDCS, provide a critical experimental tool to investigate the cognitive and emotional effects of manipulating the activity in specific neural regions implicated in affective regulation. These experimental techniques can inform the mechanisms via which tDCS exerts its therapeutic effects and also provide important insights into ways to enhance the potential benefits of tDCS.
One cognitive process that has been the focus of investigation in tDCS research is biased attention to negative emotional content. An attentional bias refers to the selective prioritisation of attention in favour of one specific class of information over another. Within the context of emotional pathology, this manifests in selective attention towards more negative/threatening information (Bar-Haim, Lamy, Pergamin, Bakermans-Kranenburg, & van Ijzendoorn, 2007; Williams, Watts, MacLeod, & Mathews, 1997). Attention bias favouring negative information has been consistently implicated in cognitive and neural models of emotional pathology as a causal process in the development and maintenance of emotional disorders (Bishop, 2007; Eysenck, Derakshan, Santos, & Calvo, 2007). It has been proposed that limbic hyperactivity combined with inadequate recruitment of frontal control serves to selectively direct attention towards negative emotional content and increase emotional vulnerability (Bishop, 2007). It follows, therefore, that increasing cortical activity in frontal areas via tDCS should serve to reduce patterns of biased attention to negative information. A number of studies have produced results consistent with this. Brunoni et al. (2014b) found that a single session of anodal tDCS to the left dorsolateral prefrontal cortex (DLPFC) reduced attentional interference of negative and positive information in an emotional Stroop task among depressed individuals. More recently, Ironside, O’Shea, Cowen, and Harmer (2016) showed that left DLPFC tDCS contributed to reductions in attention bias to fearful facial expressions in healthy participants. This finding was subsequently replicated by Heeren et al. (2017) in a sample of socially anxious individuals. Sanchez-Lopez, Vanderhasselt, Allaert, Baeken, and De Raedt (2018) also demonstrated that tDCS to the left (but not right) DLPFC resulted in faster attentional disengagement from emotional faces. A subsequent study by Ironside et al. (2019) incorporating neuroimaging showed that left DLPFC tDCS reduced biased attention to threat and decreased amygdala threat reactivity compared to sham stimulation, consistent with the role of the DLPFC in down-regulating amygdala responding to threat. Finally, Chen, Basanovic, Notebaert, MacLeod, and Clarke (2017) examined whether the effects of left DLPFC tDCS on emotional reactivity were mediated by the impact of tDCS on attention bias. The results of this study confirmed that tDCS reduced attention bias, and attention bias in turn predicted emotional reactions to negative content, consistent with a potential mediating role of attention bias. A number of studies therefore have shown that tDCS affects patterns of biased attention to threat, which may be one pathway via which neurostimulation exerts effects on emotion.
Evidence also suggests that tDCS can attenuate emotional reactivity in healthy populations. Emotional reactivity refers to the tendency to experience acute elevations in negative emotion (stress and/or dysphoria) in response to negative emotional content or stress exposure. In a recent review and meta-analysis, Smits et al. (2020) found that among 26 anodal tDCS studies, there was a relatively weak beneficial effect in reducing emotional reactivity to acute stressors or viewing negative content. It should be acknowledged that this effect was far from uniform, however, with a number of studies recording no effects of tDCS on emotional reactivity (Brunoni et al., 2013; Vierheilig, Mühlberger, Polak, & Herrmann, 2016; Voss, Ehring, & Wolkenstein, 2019). It is possible therefore that tDCS may produce larger effects on emotion reactivity if delivered in combination with the rehearsal of cognitive processes that promote the regulation of emotion.
Indeed, this possibility is in line with the hypothesis that the neuroplastic effects of tDCS may be enhanced when stimulation is combined with tasks that actively recruit neural regions targeted. Examples of this are numerous but include research in Parkinson’s patients, suggesting that tDCS in combination with physical training can improve gait and balance, whereas tDCS delivered in isolation may yield no benefit (Kaski et al., 2014). Similarly, research on working memory suggest that simultaneous tDCS and cognitive training results in greater working memory performance compared with tDCS or cognitive training alone (Martin et al., 2013; Andrews et al., 2011). It is possible therefore that the emotional effects of tDCS may be enhanced through the combination with an individual’s current active emotion processing goals.
Interestingly, an alternative line of research has shown that tDCS can reduce the perceived emotional valence of negative content and also may interact with an individual’s current emotion regulation goals to influence their perception of negative content. Emotion regulation itself refers to the processes via which individuals intentionally influence the emotions they experience, including when and how they express such emotions, and their intensity (Gross, 1998). A study by Feeser, Prehn, Kazzer, Mungee, and Bajbouj (2014) examined how emotion regulation goals interact with neurostimualtion. They found that frontal tDCS can potentially enhance the effects of intentional emotion regulation. They delivered right DLPFC tDCS (compared with sham stimulation) concurrently with an emotion regulation task in which participants were asked to switch between upregulating, downregulating, or maintaining emotional responses to negative images across trials. The study revealed that participants receiving active stimulation, compared to sham, showed both larger increases and decreases in emotional reactions to negative content in line with their current active intent to respectively up, or downregulate emotional responses. These findings are consistent with the hypothesis that frontal stimulation enhanced emotion regulation in line with an individual’s current objectives. Using a similar experimental design, Marques, Morello, and Boggio (2018) found that tDCS targeting the left ventrolateral prefrontal cortex (VLPFC; but not the DLPFC) contributed to less negative appraisals of negative emotional content. They did not, however, replicate Feeser et al.’s (2014) finding in that this was not further modified by emotion regulation goals. Thus, despite variance in the locations targeted for stimulation, findings have shown that frontal tDCS in isolation can reduce the perceived valence of negative content (Marques et al., 2018; Peña-Gómez, Vidal-Piñeiro, Clemente, Pascual-Leone, & Bartrés-Faz, 2011). Some research suggests that stimulation can interact with emotion regulation intent to influence the perceived emotionality of negative information (Feeser et al., 2014).
Interestingly, studies to date examining the interactive effects of tDCS and emotion regulation goals have consistently compared alternative regulation instructions within, rather than between participants, with experimental designs requiring participants to switch between upregulating, downregulating, and maintaining emotion intensity across trials (Feeser et al., 2014; Marques et al., 2018). While this permits examination of how alternative tDCS conditions may facilitate switching between emotion regulation instructions to influence appraisal of emotional content, it does not permit studies to examine the enduring cognitive and emotional consequences of combining tDCS with repeated practice of a single emotional regulation goal. As such, the purpose of the current study was to assess in a between-subjects design whether tDCS combined with instructed downregulation of emotional reactivity would reduce negative appraisals of aversive content, reduce patterns of biased attention, and attenuate emotional reactivity to negative content above tDCS or emotion regulation in isolation.
To achieve this, the current study incorporated four groups resulting from the combination of two between-group experimental factors of tDCS condition (Active vs. Sham) and emotion regulation instruction (Down-Regulate vs. Maintain). Identifying the most appropriate stimulation location was informed by prior studies examining the effects of tDCS on attention bias, emotional reactivity to negative content, and emotion regulation. There has been some consistency in the attention bias literature with findings showing that left DLPFC stimulation attenuates biased attention to threat (Brunoni, Boggio, et al., 2014a; Chen et al., 2017; Heeren et al., 2017; Ironside et al., 2019; Ironside et al., 2016), whereas right DLPFC stimulation does not (Sanchez-Lopez et al., 2018). Among studies that have shown significant effects of tDCS on emotional stress reactivity, five have targeted the left DLPFC, one the right DLPFC, two the right VLPFC, and two the VMPFC (Smits, Schutter, van Honk, & Geuze, 2020). As such, findings in relation to emotional stress reactivity have been shown most frequently in studies targeting the left DLPFC. The regions targeted for stimulation in studies examining the interaction of tDCS with emotion regulation has been highly heterogeneous with some studies showing enhancement of emotion regulation targeting the right DLPFC (Feeser et al., 2014), whereas others have shown no specific enhancement of emotion regulation targeting either the left or right VLPFC, or DLPFC, but general reductions in valence appraisals for stimulation to either the right or left VLPFC (Marques et al., 2018). In the absence of consistent findings from emotion regulation research, we sought to target the left DLPFC given consistent findings from research examining attention bias and emotional reactivity. Participants received either active or sham tDCS while either downregulating or maintaining their emotional reactions in response to emotionally negative images. Following this, participants completed a probe task assessment of attentional bias. Given recent findings showing that higher levels of attention bias variability may be associated with heightened emotional vulnerability (Alon, Naim, Pine, Bliese, & Bar-Haim, 2019; Zvielli, Bernstein, & Koster, 2015), it is possible that attention bias variability may be relevant to understanding how the enhancement of frontal control via tDCS (both with and without emotion regulation) impacts adaptive emotional regulation. As such, in addition to traditional measures of selective attention we also incorporated measures of attention bias variability. Finally, participants received a video viewing task assessing emotional reactivity to negative content. The intent of this task was to assess the consequent impact of tDCS delivery with or without concurrent practice of down-regulation of emotional response on spontaneous (uninstructed) emotional reactivity to negative content. If down-regulation of negative emotional content further enhances the effects of tDCS on biased cognition and emotional reactivity, then we would anticipate that individuals receiving active tDCS while consistently down-regulating their emotional responses would show less negative appraisals of negative emotional content, lower levels of attention bias and/or bias variability, and the lowest emotional reactivity to the video viewing task relative to other conditions.
Method
Participants
Participants were 116 individuals (80 females, 36 males) consisting of undergraduate students and members of the community recruited through the Curtin University School of Psychology research participant pool (Mage = 23.03, SD = 7.43). In accordance with ethics approvals for the use of tDCS, participants were eligible if they reported no history of neurological disorder, brain surgery, any active skin condition, unstable medical condition, history of migraines or faintness, any metal implants, devices, or hearing aids. Participants were made aware of these criteria before registering for the study and again upon arrival. The project was approved by the Curtin University Human Research Ethics Committee. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975 (as revised in 2000). Informed consent was obtained from all participants included in the study. A sensitivity power analysis indicated that the current sample size of 116 participants with 4 groups would provide an 80% chance of detecting approximately medium-sized effects (f = 0.26), suggesting that the current sample is adequately powered for medium effects but may be slightly underpowered for detecting smaller effects.
Emotional assessment measures
The Positive and Negative Affect Scale (PANAS; Watson, Clark, & Tellegen, 1988) was employed to assess variance in dispositional positive and negative affectivity across experimental groups. Similarly, the Depression Anxiety and Stress Scale (DASS; Lovibond & Lovibond, 1995) was administered at baseline to assess patterns of general psychological distress across groups, because patterns of biased attention and emotional reactivity are known to vary as a function of emotional vulnerability (Bar-Haim et al., 2007). Internal consistency of the subscales of these questionnaires was consistently good, with Cronbach’s alpha varying between 0.75 to 0.90 in the current study.
Transcranial direct current stimulation delivery
tDCS delivery occurred via a portable battery-powered stimulator (Chattanooga Group, United States, n.d.). Anodal stimulation targeted the left DLPFC, corresponding to F3 using the 10-20 international system. The cathode was positioned on the left superior trapezius muscle. Consistent with a number of prior studies (Chen et al., 2017; Clarke, Browning, Hammond, Notebaert, & MacLeod, 2014; Martin et al., 2013), extracephalic electrode placement was designed to ensure that any effects would not be confounded by inhibitory cortical stimulation and therefore could be more confidently attributed to anodal stimulation. Current was delivered by two 4-cm x 6-cm conductive silicone electrodes covered in saline-soaked sponges with current being ramped up/down over 1 minute. Participants in the Active condition received 2 mA of stimulation for approximately 20 minutes (see Procedure), translating to an approximate current density of 0.083 mA/cm2. Those in the Sham condition received stimulation for 1 minute, after which the current was ramped down and switched off without participant awareness. All participants were led to believe that they were receiving active stimulation. The experimenter was aware of condition allocation.
Emotion Regulation and Attentional Bias Assessment Stimuli
To examine the potential crossover effects between the emotion regulation task to patterns of attention bias, it was desirable to include stimuli that could be used in the context of both emotion regulation and the assessment of attention bias. For this purpose, we employed stimuli from the International Affective Picture System (Lang, Bradley, & Cuthbert, 1997). These stimuli are commonly used in emotion regulation tasks (Feeser et al., 2014), and while word and face stimuli are perhaps more commonly employed to assess patterns of attention bias, IAPS stimuli also have been used in the assessment of attention bias in a number of past studies (Bardeen, Tull, Daniel, Evenden, & Stevens, 2016; Clarke et al., 2017). Past literature examining attention bias in emotion has variously incorporated low arousal (depressogenic) or high arousal (anxiolytic) stimuli. While tDCS has been observed to attenuate attention bias and emotion reactivity, it is unclear whether this occurs differentially for high and low arousal stimuli. As such, to assess whether tDCS condition and/or emotion regulation condition differentially impact, and potentially interact with stimuli of different arousal levels, an equal proportion of high arousal and low arousal stimuli were selected. For the current study, emotionally negative stimuli were required for use in the emotion regulation and attention bias assessment tasks. To permit examination of whether emotion regulation condition (Down-Regulate/Maintain) could potentially impact attention bias for the stimuli encountered during the emotion regulation task, the attentional bias assessment task included two subsets of negative stimuli. One stimulus subset were negative images that had been encountered during the emotion regulation task (emotion regulation stimuli) and the other subset were novel negative stimuli that had not been encountered previously (novel stimuli). As such, a total of 60 negative stimulus images were employed across the emotion regulation and attention bias assessment tasks. Of these, 48 were used in the emotion regulation task. The attentional bias task included a subset of 12 stimuli used in the emotion regulation task and 12 novel stimuli. Stimuli were obtained from the International Affective Picture System (Lang et al., 1997). Images in the database are rated on valence (“pleasantness”) and arousal from 1 (low) to 9 (high). All negative images had valence ratings below 5 (M = 2.63, range = 1.08-3.94). Half of these negative pictures were selected on the basis of being mildly arousing (M = 5.29, range = 3.85-5.36), whereas the other half were selected on the basis of being highly arousing (M = 6.27, range = 5.52-7.35). An additional set of 24 neutral images was used only in the attention bias assessment task. These neutral images were selected on the basis of being non-negative (average ratings above 5) with values as close to 5 as possible (M = 5.32, range = 5.00–5.92).Footnote 1 To examine whether emotion regulation condition (Down-Regulate/Maintain) could impact attention bias for the stimuli encountered during the emotion regulation task, the attentional bias assessment task included 2 subsets of 12 negative stimuli, with each subset containing 6 mildly and 6 highly arousing pictures. One stimulus subset were negative images that had been encountered during the emotion regulation task (emotion regulation stimuli), and the other subset were novel negative stimuli that had not been encountered previously (novel stimuli).
Emotion regulation task
The format of the emotion regulation task was similar to other studies examining the interactive effects of tDCS and emotion regulation (Feeser et al., 2014). Participants were allocated to either the Down-Regulate or the Maintain emotional reactions condition. Those in the down-regulate condition were instructed to attempt to reduce their emotional reactions to the negative images shown by reappraising the content, with examples of potential reappraisal strategies being provided. Specifically, participants in the down-regulate condition received the following instructions:
“When you view the following images, we would like you to try and feel less negative about the picture by trying to change the meaning of it. That means you think of something to tell yourself about the picture that helps you feel less negative about it. So, for example, you could tell yourself something about the outcome so that whatever is going on will soon be resolved, or that help is on the way. Or you could try and view the situation in an impartial way, similar to how a doctor might.
You could also focus on a detail of the situation that may not be as bad as it first seemed. But we want you to stay focused on the picture and not think of random things that make you feel better, but rather to change something about the way you view the picture that helps you to feel less negative about it.”
Alternatively, those in the Maintain condition instead received the following instructions:
“When you view the following images, we would like you to try and maintain your emotional reaction to what you see in the picture without attempting to change it. Try to stay focused on the image and allow yourself to experience your natural emotional reaction to the picture without suppressing how you feel about it.”
On each trial of the emotion regulation task, a fixation point was initially displayed for 2,000 ms in the centre of the screen, followed by the negative stimulus image presented on a white background 165 x 165 mm in size for 8,000 ms. Following the offset of the image, a screen was presented with the question “How did you feel viewing that image?” with a 12-cm line presented below, with the anchors “Not negative at all” to “Extremely negative.” Participants then marked a point along this line to indicate their emotional reaction to the image. This yielded a value from 0-12 with higher scores representing more negative emotional reactions. The task consisted of 48 trials in total, with each image being presented once in random order.
Attention bias assessment task
To assess the impact of experimental conditions on biased attention, we employed a variant of the attentional probe assessment task (MacLeod, Mathews, & Tata, 1986). On each trial of this task, a black fixation cross was displayed for 500 ms followed by a negative-neutral image pair, presented side by side on a white background, each occupying a space 60 mm x 78 mm, aligned on the horizontal axis, separated by 65 mm. These stimuli were presented for 500 ms, after which a small grey arrow 5 mm in length appeared in the location vacated by one of the two stimuli. Participants were required to indicate whether the arrow pointed up or down by pressing the corresponding up or down button on the keyboard. The participant response cleared the screen, and the next trial started after a 500-ms delay. If participants recorded an incorrect response, the word “incorrect” was displayed for 3 seconds before continuing to the next trial. This was designed to encourage correct responding. Negative/neutral picture position and probe position were counter-balanced across trials such that probes appeared with equal frequency in the location of negative pictures (“probe-negative” trials) and in the location of the neutral pictures (“probe-neutral” trials). A total of 96 trials were delivered in four randomised blocks of 24 trials. Of each block of 24, 12 contained high arousal stimuli, and 12 contained low arousal stimuli. Of the high and low arousal negative stimuli, half were “old” having been presented during the emotion regulation task, and 12 were “novel” having not been previously encountered.
Emotional reactivity task
The effect of experimental condition on spontaneous (uninstructed) emotional reactivity was assessed in response to four negative video clips. Participants were not supplied with any direction regarding the processing of emotional content in the videos. Two of the clips depicted high arousal negative content (e.g., fleeing armed militia – Blood Diamond), and two depicted low-arousal negative content (e.g., bedside death scene – The Champ). Each video lasted approximately 2 minutes and were delivered consecutively with no interruption. Emotional reactivity to the video viewing task was assessed using two visual analogue mood scales delivered before and after the viewing of all videos. These consisted of a 12-cm line with the anchors “Happy-Sad,” and “Relaxed-Anxious.” Participants marked the point on the line corresponding to their current mood to yield separate measures of dysphoric and anxious mood with a score from 0 to 12. For each measure, the pre-video mood scale was subtracted from the post-video mood scale to yield an index of change in dysphoric and anxious mood. Higher scores indicated greater increase in negative mood.
tDCS manipulation check
To assess participants’ awareness of allocation to tDCS conditions, at the conclusion of the study, participants were provided a question informing them that “in the current study, there were two different neurostimulation conditions, one that delivered active stimulation, and one that did not. Please indicate the condition that you believe you received.” Participants then recorded whether they believed they had received “active” or “nonactive” stimulation.
Procedure
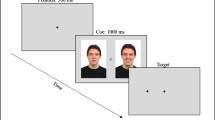
Upon arrival, participants were provided information on the requirements of the study and given opportunity to ask questions before providing their written, informed consent. Participants were randomly assigned to one of the four experimental conditions using a predetermined random assignment of participant numbers. Fig. 1 provides a summary of experimental procedures in the study. Participants initially completed baseline questionnaires and were then fitted with tDCS equipment. Stimulation was then initiated according to their assigned condition. After 6 minutes of sham/active stimulation, the emotion regulation task commenced. Participants initially received instructions on the completion of the task, including emotion regulation instructions, according to their assigned condition. Participants then completed all 48 trials of the emotion regulation task, after which tDCS stimulation was ceased. This corresponded to approximately 20 minutes in total of stimulation. A subset of participants (n = 76) also completed a brief (5 min) word-association reaction time task at this point as part of an alternative study. Participants then completed the attentional probe assessment task, including 16 practice trials using neutral stimuli only. Following this, participants completed the emotional reactivity assessment task, which involved viewing the four video segments immediately preceded and followed by the completion of mood scales. At the completion of this, participants were offered the opportunity to view two positive videos to promote mood recovery and were then delivered the tDCS manipulation check question and were debriefed regarding the purpose of the study.
Order and timing of experimental procedures. Participants initially completed baseline questionnaires (BQ) before initiation of tDCS/sham stimulation. After 6 minutes of active/sham stimulation, the emotion regulation task commenced in line with the allocated condition (down-regulate/maintain). Participants then completed the attention probe assessment task, followed by the emotion reactivity assessment task (video viewing) which was preceded and followed by mood ratings
Results
Baseline group characteristics
A one-way ANOVA on baseline emotional measures across the four experimental groups revealed no significant differences in any of the DASS or PANAS subscales (largest F = 1.16, smallest p = 0.328). Chi square analysis also showed no significant differences across experimental groups in gender ratio, χ2 (3, 115) = 1.17, p = 0.760. Likewise, analysis of participants’ response to the tDCS manipulation check question showed that participants could not reliably identify whether they had been allocated to the Active or Sham the condition χ2 (1, 116) = 1.77, p = 0.230, with 75% of those in the Active tDCS condition and 63% of those in the Sham condition believing they were receiving active tDCS.Footnote 2 Descriptive characteristics of groups are provided in Table 1.
Impact of tDCS and emotion regulation strategy on the emotional regulation task
To examine the effect of experimental condition on responses to the images encountered in the emotion regulation task, a 2 x 2 x 2 repeated measures ANOVA was conducted with the two between groups factors of tDCS Condition (Active vs. Sham stimulation) and Emotion Regulation Condition (Down-Regulate vs. Maintain)—the within subjects factor of Stimulus Arousal Level (High arousal vs. Low arousal) and the dependent variable of self-report emotional reactions to stimulus images. A significant main effect of Stimulus Arousal Level was observed F(1, 112) = 461.67, p < 0.001, Ƞp2 = 0.81, showing that high arousal stimuli were consistently rated as more negative (M = 6.26, SD = 2.41) compared with low arousal stimuli (M = 4.43, SD = 2.07). There also was a main effect of Emotion Regulation Condition, F(1, 112) = 20.621, p < 0.001, Ƞp2 = 0.155, showing that those in the Down-Regulate condition consistently rated all stimuli as less negative (M = 4.50, SD = 2.07) compared with those in the Maintain condition (M = 6.22, SD = 2.09). No other significant main effects or interactions were observed (all Fs < 2.07, all ps > 0.153), indicating that tDCS did not have the expected differential effects on either negative emotion experience or participants’ capacity to downregulate negative emotions.
Impact of tDCS and emotion regulation on attention bias and attention bias variability
Preparation of response-time data
Accuracy on the attentional probe assessment task was consistently high, with no participant recording an accuracy rate below 85% (M = 98.01%, SD = 2.06%). Probe reaction time data was prepared by initially excluding response times below 200 ms and above 2,000 ms, any incorrect responses, and response times falling 3 median absolute deviations (Leys, Ley, Klein, Bernard, & Licata, 2013) from each participant’s own mean reaction time, resulting in the exclusion of 3.67% of trials (the same response time and accuracy exclusions were applied across all bias index computation methodologies). For the standard average index measure of attention bias, four separate indices were computed for the combination of high and low arousal stimuli, and for both old (encountered during the emotion regulation task) and novel (not previously encountered) stimuli. Each of these were computed by subtracting probe-threat trials from probe-neutral trials with higher scores representing greater attention bias to threat. These traditional indices of attention bias we refer to as the average index measure of attention bias (ABAveIndex). Examination of standardised scores for each bias index revealed a small number (n = 3) that fell more than 3 SD from the group mean. These participants were excluded from analyses involving these index measures.
In addition to the traditional measure of attention bias, we also computed two recently developed measures of attention bias variability. The first of these measures was based on the method employed by Naim et al. (2015). This methodology involves the initial computation of multiple attention bias index scores for each participant, subtracting reaction times of individual probe-threat trials from reaction times of individual probe-neutral trials according to their temporal appearance in the task. Moving mean attention bias index scores are then computed for groups of 10 index scores (i.e., for index scores 1-10, 2-11, 3-12, etc.). The standard deviation of these average bias scores is then calculated and divided by the participant’s overall mean reaction time forming the final measure of bias variability, which we refer to as the moving average measure of attention bias variability (ABVMovAve). One participant’s score exceeded 3 SD from the group mean for this measure and was excluded from analyses involving this index.
The second measure of attention bias variability was an adapted version of the methodology employed by Zvielli et al. (2015). For this approach, we employed a similar initial step of pairing probe-threat and probe-neutral trials in order of appearance on the task to compute multiple bias index scores. The difference between each index score and the subsequent index is then computed (i.e., difference between index 1-2, 2-3, 3-4, etc.). The average of the normalised values of these differences then provides the final measure of attention bias variability, which we refer to as the trial-level bias score measure of attention bias variability (ABVTLBS). In examining the distribution of values on this measure, three participants’ scores fell beyond 3 SD from the group mean and were excluded from analyses involving this measure. As three alternative measures relating to attention bias were generated from this single set of reaction time data, it was deemed appropriate to implement Bonferroni correction for multiple comparisons. As such, the adjusted alpha level for analyses involving attention bias was α = 0.012.
An examination of correlations between each of these attention bias measures and each subscale of the emotional assessment measures at baseline (DASS and PANAS) revealed no significant relationships (all rs < 0.14, all ps > 0.13).
Effect of tDCS and emotion regulation condition on average attention bias
Descriptive data for the ABAveIndex measures across conditions is provided in Table 2. To examine the effect of tDCS and emotion regulation conditions on average biased attention, we conducted a 2 x 2 x 2 x 2 repeated measures ANOVA with the between-subjects factors of tDCS Condition, Emotion Regulation Condition, and the within-subject factors of Stimulus Arousal Level (High vs. Low arousal) and Stimulus Familiarity (Old vs. Novel stimuli). Not a single main effect or interaction was observed to be significant for this analysis (all Fs < 2.77, all ps > 0.099), indicating that neither tDCS nor emotion regulation instructions affected the traditional average attention bias index.
As some past findings have shown that attention bias may only be exhibited at high levels of stimulus threat intensity (Wilson & MacLeod, 2003), a post-hoc analysis was run to examining high and low arousal stimuli separately. Whereas no effect of tDCS condition was shown for the low arousal stimuli (F(1, 109) = 0.45, p = 0.67), there was a slight trend toward a significant main effect of tDCS condition for higher arousal stimuli F(1, 109) = 3.60, p = 0.061, ηp2 = 0.032). Those in the Sham condition selectively attended toward these stimuli on average (M = 6.57, SD = 32.80), whereas those in the Active tDCS condition showed avoidance of these stimuli (M = −5.12, SD = 32.61). It should be noted however that this “trend” appears less compelling when considering the adjusted α of 0.012.
Effect of tDCS and emotion regulation condition on attention bias variability
Descriptive data for the ABVMovAve and the ABVTLBS measures of attention bias variability are provided in Table 2. Two separate 2 x 2 ANOVAs were conducted for these two dependent variables involving tDCS Condition and Emotion Regulation Condition as between-subject factors. The analysis involving ABVMovAve as the dependent variable showed no significant main effects or interactions (all Fs < 0.32, all ps > 0.575). The analysis with ABVTLBS as the dependent measure likewise showed no significant main effects or interactions (all Fs < 1.54, all ps > 0.218).
Impact of tDCS and emotion regulation on the emotional reactivity task
To examine the effects of experimental condition on emotional reactivity, we conducted a 2 x 2 x 2 repeated measures ANOVA with the two between-subject factors of tDCS Condition and Emotion Regulation Condition and the within subjects factor of Scale Type (anxiety vs. dysphoria). This analysis revealed a main effect of Scale Type, F(1, 112) = 28.49, p < 0.001, ηp2 = 0.203, whereby participants showed larger increases in dysphoric mood (M = 3.72, SD = 2.98) compared with anxious mood (M = 2.06, SD = 2.98). A main effect of tDCS Condition also was observed, F(1, 112) = 5.59, p = 0.020, ηp2 = 0.05, showing that those in the Active tDCS condition (M = 2.34, SD = 2.66) showed smaller increases in negative emotional reactions overall compared with those in the Sham tDCS condition (M = 3.44, SD = 2.66). This effect is consistent with tDCS attenuating reactions to negative emotional content. No additional main effects or interactions were significant, all Fs < 0.96, all ps > 0.329.
Discussion
The current study sought to investigate whether active versus sham left DLPFC tDCS delivered online with either intentional down-regulation or maintenance of emotional reactions would differentially influence: 1) negative appraisals of aversive content during emotional regulation; 2) reduce patterns of biased attention to negative information; and 3) the spontaneous emotional reactivity to negative content. We found no evidence that tDCS had a general effect of reducing negative appraisals of emotionally negative stimuli, nor evidence that tDCS interacted with emotion regulation condition to enhance the effects of downregulation of emotional reactivity. There also were no significant effects of tDCS condition or emotion regulation condition on different indices of attention bias. The single clear finding to emerge from this study was that active tDCS led to less negative emotional reactivity compared with sham tDCS, as revealed by changes in mood in response to the video viewing task. As such, the present findings support the effect of frontal tDCS in reducing negative emotional reactivity.
The absence of an effect of tDCS reducing patterns of attention bias differs from a number of other recent studies that have shown such an effect (Chen et al., 2017; Heeren et al., 2017; Ironside et al., 2019; Ironside et al., 2016; Sanchez-Lopez et al., 2018). A number of differences between experimental designs could potentially account for this. One possibility is that greater variance between conditions due to the between-group design may have contributed to greater noise, reducing the likelihood of detecting such an effect. Indeed, the majority of recent studies to demonstrate an effect of tDCS on attention bias have used within-subject crossover designs (Heeren et al., 2017; Ironside et al., 2019; Sanchez-Lopez et al., 2018), which can reduce additional noise present in between-group designs. However, the higher participant numbers in the current study compared to these, and the fact that a number of other between-group studies also have detected tDCS-attention bias effects (Chen et al., 2017; Ironside et al., 2016) suggests that the additional noise associated with a between-groups design is unlikely to be a single cause of this.
An additional difference concerns the type of stimuli used. The majority of past studies have employed disgust or fearful face stimuli in assessments of attention bias (Heeren et al., 2017; Ironside et al., 2019; Ironside et al., 2016; Sanchez-Lopez et al., 2018), with another using simultaneous competing threat-neutral videos (Chen et al., 2017). It is possible, therefore, that the greater complexity of the IAPS stimuli in the current study (e.g., scenes of war or conflict) could mean that valence was less rapidly registered. Using stimuli that permit the rapid identification of valence, and/or the use of longer stimulus exposure durations, may be an important feature for studies seeking to measure the effects of tDCS on attention bias.
A final possibility for the absence of tDCS effects on attentional bias relates to stimulus intensity. Some early attention bias studies highlighted that among samples with low or average levels of anxiety, an attentional bias is only exhibited at high levels of stimulus threat intensity (Wilson & MacLeod, 2003). Not having stimuli of sufficient intensity to evoke a general pattern of bias (for tDCS to then mitigate) may have reduced the likelihood of detecting an effect of tDCS on attentional bias. Indeed, as reported in the post-hoc analysis examining the pattern of attention bias for high and low arousal stimuli separately, no effect of tDCS condition was shown for the low arousal stimuli with a slight trend toward a significant main effect of tDCS condition for higher arousal stimuli, whereby those in the Sham condition selectively attended toward these stimuli on average while those in the Active tDCS condition showed avoidance of these stimuli. While no conclusions should be drawn on the basis of this post-hoc trend, it is possible that more intense stimuli and/or a greater number of trials containing high arousal stimuli may have yielded a significant main effect of tDCS on attention bias. This may be particularly the case given the low reliability of attention probe assessment tasks (Price et al., 2015).
The present study found no evidence for an association between any of the attention bias measures employed and baseline measures of anxiety or positive and negative affectivity. In addition to potential noted issues relating to the stimuli used in the current design, it is possible that the present study may not have been sufficiently powered to detect correlations involving such effects. As has been noted, attention bias to threat tends to be observed in between-subjects designs comparing those higher and lower in emotional pathology although is less commonly demonstrated in correlational designs (MacLeod, Grafton, & Notebaert, 2019). Indeed, recent findings, which included each of the measures of attention bias examined in the current study (although with the use of word stimuli) with a larger sample of 195 participants, found significant though small associations with anxiety (r = 0.254 to 0.183; Clarke et al., 2020a). This highlights the possibility that the combination of power and stimuli may have prevented the detection of such anticipated associations in the current study.
The current study also found no evidence that frontal tDCS interacted with emotion regulation intention to influence perceptions of negative stimuli during the emotion regulation task. This finding contrasts with that of Feeser et al. (2014) who found that tDCS enhanced the down (and up)-regulation of the perceptions of emotional content. One possibility for the absence of this effect is that the current study was potentially underpowered to detect smaller effects. Indeed, a post-hoc power analysis indicated that with the observed main effect of tDCS on emotional reactivity of ηp2 = 0.05 (f = 0.23), the current sample of 116 achieved a power of 69%, suggesting that the study may have been underpowered to detect smaller effects. While the number or participants in each condition (n = 27-30) is broadly comparable, and perhaps slightly higher than other similar tDCS studies (e.g., Feeser et al., 2014 - 21/condition), higher power is obviously desirable and represents a limitation of the current study.
Another possibility for the lack of this effect is the difference between stimulation sites in the current study (left DLPFC) and Feeser et al. (2014; Right DLPFC). However, as with the current study, Marques et al. (2018), who employed a larger sample (n = 45/condition) also found no evidence that tDCS targeting the left or right DLPFC, or left or right VLPFC resulted in the enhancement of intentional emotion regulation capacity. Given these recent null findings, and the fact that evidence of tDCS-enhancement of intentional emotion regulation has, to our knowledge, been demonstrated only in a single study (Feeser et al., 2014) with a reasonably small sample (n = 21), the evidence that tDCS can enhance emotion regulation appears equivocal at present.
While the present null findings regarding the tDCS-enhancement of emotion regulation are consistent with other recent findings, it is relevant to consider the possibility that the absence of such effects may have been due to inadequate modulation of neural activity in the region targeted for stimulation. Specifically, it is possible that the use of an extracephalic reference electrode could have contributed to this with the greater distance between the anode and cathode potentially reducing the current density at the stimulation site. However, a number of findings suggest that such electrode placements can successfully modulate neural activity, with consequent effects on related cognitive and emotional processes. For example, studies have shown that the use of such extracephalic electrode placement in combination with anodal DLPFC stimulation yields effects on biased attention (Chen et al., 2017; Clarke et al., 2014) and can enhance behavioural effects interactively with current cognitive goals (Martin et al., 2013). Recent findings systematically comparing cortical and extracephalic cathode placement on basic enhancement of cortical excitability (motor evoked potentials) also have shown that both arrays enhance excitability and is maintained for 60 minutes after stimulation (Tatemoto, Yamaguchi, Otaka, Kondo, & Tanaka, 2013). As such, it seems unlikely that the electrode array employed in the present study did not lead to sufficient enhancement of cortical excitability.
While methodological differences between studies represent one reason for inconsistencies in findings, it also is possible that differences in the way that individuals respond to tDCS, and neurostimulation more generally could contribute to such inconsistency. For example, it has been recognised that there exists considerable variance in the response to tDCS for depression, with large interindividual variability in clinical outcomes noted (Brunoni et al., 2016). Rather than reflecting general “noise” between individuals, research is beginning to show that such variance in responsivity to tDCS may be meaningfully associated with neurochemical balances. A recent study by Filmer, Ehrhardt, Bollmann, Mattingley, and Dux (2019) showed that the baseline balance of excitatory and inhibitory neurochemicals (GABA and glutamate) predicted the extent to which tDCS impacted the acquisition of learning on a forced-choice task (Barron et al., 2016). This follows earlier work showing that GABA responsiveness may predict the effects of tDCS on learning. Some initial findings also highlight that it may be possible to predict the potential therapeutic effects of tDCS. A study by Bulubas et al. (2019) indicated that measures of neural structure (medial prefrontal grey matter volume) were associated with subsequent effects of tDCS on depression. Other findings have shown that degree of neural activation during a baseline working-memory task subsequently predicted degree of symptom improvement in tDCS treatment for depression. Such early findings provide encouragement for the potential ability to identify factors that account for variance in the impact of tDCS on emotion in the future.
The present study provided evidence that left DLPFC tDCS attenuated negative emotional reactivity to the aversive content depicted in the video viewing task. These results are consistent with previous findings suggesting that tDCS can attenuate emotional reactivity via frontal stimulation. Specifically, Rêgo et al. (2015) examined whether left DLPFC tDCS would influence emotional reactions to video content depicting painful situations. Their findings showed that left DLPFC tDCS attenuated elevations in ratings of negative valence and emotional arousal in response to viewing this content compared to right DLPFC tDCS and sham tDCS conditions. More generally, the present findings showing a small effect of tDCS on the reduction of emotional reactivity are consistent with the recent meta-analysis by Smits et al. (2020). This showed that across 26 studies, tDCS in healthy samples has a significant, although weak, effect on reducing emotional stress reactivity. While consistent with these past findings, a limitation of the present study is the exclusive reliance on self-report to assess emotional reactivity. Future research might consider the inclusion of additional measures of current emotional state, such as heart rate variability and skin conductance, to corroborate self-report mood measures and also should seek to replicate the current pattern of effects in a larger sample.
A further limitation in the assessment of emotional reactivity was the fact that the current design did not enable the separate examination of the emotional effects of high arousal (threat/danger) and low arousal (sadness/loss) video content. While anxiety and sadness were separately indexed before and after all videos, the delivery of the clips within a single block did not allow the separate assessment of the effects on anxious compared with dysphoric mood. While the assessment of mood after each video clip would have been impractical due to sequence effects, it would be useful for future research to examine the emotional effects of tDCS for dysphoric versus anxiolytic content by delivering different types of stressor between groups.
Finally, the video assessment task also exclusively focused on emotional reactivity while not addressing the subsequent recovery from the emotional stressor. As psychopathology is commonly associated with greater emotional reactivity to negative and/or threatening experiences (Chen, Clarke, MacLeod, Hickie, & Guastella, 2016), experimental research often focuses on this reactivity component of emotional change as a proxy for understanding the impact of cognitive and neural processes on emotion. However, there is increasing recognition that individual differences in emotional recovery following exposure to a negative experience/content can account for unique variance in emotional distress above and beyond emotional reactivity alone (Boyes, Carmody, Clarke, & Hasking, 2017; Boyes, Clarke, & Hasking, 2020; Ripper, Boyes, Clarke, & Hasking, 2018). Indeed, a recent study examining the emotional effects of tDCS in relation to intentional worry indicated that active left DLPFC stimulation not only impacted emotional reactivity, but facilitated subsequent reductions in negative emotion compared with sham (Clarke, Sprlyan, Hirsch, Meeten, & Notebaert, 2020b). As such, it would be relevant for future research to examine emotional reactivity in response to negative emotional experiences, as well as subsequent recovery following the cessation of such an experience/stressor, to inform whether tDCS facilitates one or both of these processes.
Conclusions
The present study sought to examine the effects of tDCS and emotion regulation on perception of emotional stimuli, biased attention, and emotional reactivity to negative content. There was no evidence that tDCS and emotion regulation strategy interacted to influence the perception of negative stimuli. We were not able to confirm an effect of tDCS attenuating biased attention for negative information, and we found no evidence in support of the effect of tDCS or emotion regulation on attention bias variability. However, we did find clear evidence that tDCS contributed to less elevation in negative mood in response to a stressor as compared to sham stimulation. As such, the present findings are consistent with other studies that have found no evidence to support tDCS enhancement of emotional regulation and supports the potential of left frontal tDCS to reduce emotional reactivity to negative content.
Notes
Low arousal negative IAPS images: 2141, 2205, 2276, 2301, 2312, 2399, 2456, 2682, 2692, 2718, 2750, 2799, 2900.1, 3181, 3300, 3301, 4621, 6825, 7520, 9000, 9010, 9180, 9186, 9280, 9295, 9331, 9419, 9426, 9430, 9435, 9584, 9594, 9610, 9922, 9926, 9927.
High arousal negative IAPS images: 1304, 2703, 3103, 3120, 3180, 3220, 3350, 3530, 3550, 6212, 6230, 6312, 6510, 6520, 6560, 6838, 8485, 9050, 9160, 9163, 9250, 9254, 9400, 9410, 9412, 9413, 9414, 9423, 9424, 9425, 9429, 9600, 9623, 9908, 9911, 9921.
Neutral IAPS images: 2102, 2107, 2384, 5390, 5471, 5520, 5533, 5731, 5740, 7000, 7003, 7004, 7026, 7052, 7081, 7090, 7233, 7300, 7490, 7500, 7512, 7547, 7550, 7830.
A number of participants in the Sham condition also disclosed that they had believed they were receiving active tDCS throughout the study until after they had been alerted to the alternative conditions by the question.
References
Alon, Y., Naim, R., Pine, D. S., Bliese, P. D., & Bar-Haim, Y. (2019). Validity of Attention Bias Variability Indices for Posttraumatic Stress Disorder Research: Evidence From Patient Data. 32(5), 791-798. doi:https://doi.org/10.1002/jts.22443
Andrews, S. C., Hoy, K. E., Enticott, P. G., Daskalakis, Z. J., & Fitzgerald, P. B. (2011). Improving working memory: the effect of combining cognitive activity and anodal transcranial direct current stimulation to the left dorsolateral prefrontal cortex. Brain stimulation, 4(2), 84–89.
Bardeen, J. R., Tull, M. T., Daniel, T. A., Evenden, J., & Stevens, E. N. (2016). A Preliminary Investigation of the Time Course of Attention Bias Variability in Posttraumatic Stress Disorder: The Moderating Role of Attentional Control. Behaviour Change, 33(2), 94-111. doi:https://doi.org/10.1017/bec.2016.5
Bar-Haim, Y., Lamy, D., Pergamin, L., Bakermans-Kranenburg, M. J., & van Ijzendoorn, M. H. (2007). Threat-related attentional bias in anxious and nonanxious individuals: A meta-analytic study. Psychological Bulletin, 133(1), 1-24. https://doi.org/10.1037/0033-2909.133.1.1
Barron, H. C., Vogels, T. P., Emir, U. E., Makin, T. R., O’Shea, J., Clare, S., … Behrens, T. E. J. (2016). Unmasking Latent Inhibitory Connections in Human Cortex to Reveal Dormant Cortical Memories. Neuron, 90(1), 191-203. doi:https://doi.org/10.1016/j.neuron.2016.02.031
Bishop, S. J. (2007). Neurocognitive mechanisms of anxiety: an integrative account. Trends in Cognitive Sciences, 11(7), 307-316. doi:https://doi.org/10.1016/j.tics.2007.05.008
Boyes, M. E., Carmody, T. M., Clarke, P. J. F., & Hasking, P. A. (2017). Emotional reactivity and perseveration: Independent dimensions of trait positive and negative affectivity and differential associations with psychological distress. Personality and Individual Differences, 105, 70-77. https://doi.org/10.1016/j.paid.2016.09.025
Boyes, M. E., Clarke, P. J. F., & Hasking, P. A. (2020). Relationships between dispositional and experimentally elicited emotional reactivity, intensity, and perseveration. Personality and Individual Differences, 152, 109573. https://doi.org/10.1016/j.paid.2019.109573
Brunoni, A. R., Boggio, P. S., De Raedt, R., Benseñor, I. M., Lotufo, P. A., Namur, V., … Vanderhasselt, M. A. (2014a). Cognitive control therapy and transcranial direct current stimulation for depression: A randomized, double-blinded, controlled trial. Journal of Affective Disorders, 162, 43-49. doi:https://doi.org/10.1016/j.jad.2014.03.026
Brunoni, A. R., Moffa, A. H., Fregni, F., Palm, U., Padberg, F., Blumberger, D. M., … Alonzo, A. (2016). Transcranial direct current stimulation for acute major depressive episodes: meta-analysis of individual patient data. The British Journal of Psychiatry, 208(6), 522-531.
Brunoni, A. R., Vanderhasselt, M.-A., Boggio, P. S., Fregni, F., Dantas, E. M., Mill, J. G., … Benseñor, I. M. (2013). Polarity- and valence-dependent effects of prefrontal transcranial direct current stimulation on heart rate variability and salivary cortisol. Psychoneuroendocrinology, 38(1), 58-66. https://doi.org/10.1016/j.psyneuen.2012.04.020
Brunoni, A. R., Zanao, T. A., Vanderhasselt, M. A., Valiengo, L., Oliveira, J. F., Boggio, P. S., … Fregni, F. (2014b). Enhancement of Affective Processing Induced by Bifrontal Transcranial Direct Current Stimulation in Patients With Major Depression. Neuromodulation: Technology at the Neural Interface, 17(2), 138-142. doi:https://doi.org/10.1111/ner.12080
Bulubas, L., Padberg, F., Bueno, P. V., Duran, F., Busatto, G., Amaro, E., … Brunoni, A. R. (2019). Antidepressant effects of tDCS are associated with prefrontal gray matter volumes at baseline: Evidence from the ELECT-TDCS trial. Brain stimulation, 12(5), 1197-1204. https://doi.org/10.1016/j.brs.2019.05.006
Chattanooga Group, H. (United States) (n.d.) . Chattanooga Dual Channel Iontophoresis System. Hixon, TN, USA: Encore Medical.
Chen, N. T. M., Basanovic, J., Notebaert, L., MacLeod, C., & Clarke, P. J. F. (2017). Attentional bias mediates the effect of neurostimulation on emotional vulnerability. Journal of Psychiatric Research, 93, 12-19. doi:https://doi.org/10.1016/j.jpsychires.2017.05.008
Chen, N. T. M., Clarke, P. J. F., MacLeod, C., Hickie, I. B., & Guastella, A. J. (2016). Aberrant Gaze Patterns in Social Anxiety Disorder: An Eye Movement Assessment during Public Speaking. Journal of Experimental Psychopathology, 7(1), 1-17. doi:https://doi.org/10.5127/jep.040313
Clarke, P. J. F., Branson, S., Chen, N. T. M., Van Bockstaele, B., Salemink, E., MacLeod, C., & Notebaert, L. (2017). Attention bias modification training under working memory load increases the magnitude of change in attentional bias. Journal of Behavior Therapy and Experimental Psychiatry, 57, 25-31. doi:https://doi.org/10.1016/j.jbtep.2017.02.003
Clarke, P. J. F., Browning, M., Hammond, G., Notebaert, L., & MacLeod, C. (2014). The Causal Role of the Dorsolateral Prefrontal Cortex in the Modification of Attentional Bias: Evidence from Transcranial Direct Current Stimulation. Biological Psychiatry, 76(12), 946–952. doi:https://doi.org/10.1016/j.biopsych.2014.03.003
Clarke, P. J. F., Marinovic, W., Todd, J., Basanovic, J., Chen, N. T. M., & Notebaert, L. (2020a). What is attention bias variability? Examining the potential roles of attention control and response time variability in its relationship with anxiety. PsyArXiv. https://doi.org/10.31234/osf.io/ynp7q
Clarke, P. J. F., Sprlyan, B. F., Hirsch, C. R., Meeten, F., & Notebaert, L. (2020b). tDCS increases anxiety reactivity to intentional worry. Journal of Psychiatric Research, 120, 34-39. https://doi.org/10.1016/j.jpsychires.2019.10.013
Eysenck, M. W., Derakshan, N., Santos, R., & Calvo, M. G. (2007). Anxiety and cognitive performance: Attentional control theory. Emotion, 7(2), 336-353. https://doi.org/10.1037/1528-3542.7.2.336
Feeser, M., Prehn, K., Kazzer, P., Mungee, A., & Bajbouj, M. (2014). Transcranial Direct Current Stimulation Enhances Cognitive Control During Emotion Regulation. Brain stimulation, 7(1), 105-112.
Filmer, H. L., Ehrhardt, S. E., Bollmann, S., Mattingley, J. B., & Dux, P. E. (2019). Accounting for individual differences in the response to tDCS with baseline levels of neurochemical excitability. Cortex, 115, 324-334. https://doi.org/10.1016/j.cortex.2019.02.012
Gross, J. J. (1998). The emerging field of emotion regulation: An integrative review. Review of General Psychology, 2(3), 271-299. doi:https://doi.org/10.1037/1089-2680.2.3.271
Heeren, A., Billieux, J., Philippot, P., De Raedt, R., Baeken, C., de Timary, P., … Vanderhasselt, M.-A. (2017). Impact of transcranial direct current stimulation on attentional bias for threat: a proof-of-concept study among individuals with social anxiety disorder. Social Cognitive and Affective Neuroscience, 12(2), 251-260. doi:https://doi.org/10.1093/scan/nsw119
Ironside, M., Browning, M., Ansari, T. L., Harvey, C. J., Sekyi-Djan, M. N., Bishop, S. J., … O’Shea, J. (2019). Effect of Prefrontal Cortex Stimulation on Regulation of Amygdala Response to Threat in Individuals With Trait Anxiety: A Randomized Clinical Trial. Jama Psychiatry, 76(1), 71-78. doi:https://doi.org/10.1001/jamapsychiatry.2018.2172
Ironside, M., O’Shea, J., Cowen, P. J., & Harmer, C. J. (2016). Frontal Cortex Stimulation Reduces Vigilance to Threat: Implications for the Treatment of Depression and Anxiety. Biological Psychiatry, 79(10), 823-830. doi:https://doi.org/10.1016/j.biopsych.2015.06.012
Kaski, D., Dominguez, R., Allum, J., Islam, A., & Bronstein, A. (2014). Combining physical training with transcranial direct current stimulation to improve gait in Parkinson’s disease: a pilot randomized controlled study. Clinical Rehabilitation, 28(11), 1115–1124. https://doi.org/10.1177/0269215514534277.
Lang, P. J., Bradley, M. M., & Cuthbert, B. N. (1997). International affective picture system (IAPS): Technical manual and affective ratings. NIMH Center for the Study of Emotion and Attention, 39-58.
Leys, C., Ley, C., Klein, O., Bernard, P., & Licata, L. (2013). Detecting outliers: Do not use standard deviation around the mean, use absolute deviation around the median. Journal of Experimental Social Psychology, 49(4), 764-766. https://doi.org/10.1016/j.jesp.2013.03.013
Loo, C. K., Alonzo, A., Martin, D., Mitchell, P. B., Galvez, V., & Sachdev, P. (2012). Transcranial direct current stimulation for depression: 3-week, randomised, sham-controlled trial. The British Journal of Psychiatry, 200(1), 52-59. doi:https://doi.org/10.1192/bjp.bp.111.097634
Lovibond, P., & Lovibond, S. (1995). The structure of negative emotional states: Comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behaviour Research and Therapy, 33(3), 335-343.
MacLeod, C., Grafton, B., & Notebaert, L. (2019). Anxiety-Linked Attentional Bias: Is It Reliable? Annual Review of Clinical Psychology, 15(1), 529-554. doi:https://doi.org/10.1146/annurev-clinpsy-050718-095505
MacLeod, C., Mathews, A., & Tata, P. (1986). Attentional bias in emotional disorders. Journal of Abnormal Psychology, 95(1), 15-20.
Marques, L. M., Morello, L. Y. N., & Boggio, P. S. (2018). Ventrolateral but not Dorsolateral Prefrontal Cortex tDCS effectively impact emotion reappraisal – effects on Emotional Experience and Interbeat Interval. Scientific Reports, 8(1), 15295. doi:https://doi.org/10.1038/s41598-018-33711-5
Martin, D. M., Liu, R., Alonzo, A., Green, M., Player, M. J., Sachdev, P., & Loo, C. K. (2013). Can transcranial direct current stimulation enhance outcomes from cognitive training? A randomized controlled trial in healthy participants. International Journal of Neuropsychopharmacology, 16(9), 1927-1936. doi:https://doi.org/10.1017/s1461145713000539
Naim, R., Abend, R., Wald, I., Eldar, S., Levi, O., Fruchter, E., … Bar-Haim, Y. (2015). Threat-Related Attention Bias Variability and Posttraumatic Stress. American Journal of Psychiatry, 172(12), 1242-1250. doi:https://doi.org/10.1176/appi.ajp.2015.14121579
Palm, U., Schiller, C., Fintescu, Z., Obermeier, M., Keeser, D., Reisinger, E., … Padberg, F. (2012). Transcranial direct current stimulation in treatment resistant depression: A randomized double-blind, placebo-controlled study. Brain stimulation, 5(3), 242-251. https://doi.org/10.1016/j.brs.2011.08.005
Peña-Gómez, C., Vidal-Piñeiro, D., Clemente, I. C., Pascual-Leone, Á., & Bartrés-Faz, D. (2011). Down-regulation of negative emotional processing by transcranial direct current stimulation: effects of personality characteristics. PLoS ONE, 6(7), e22812.
Price, R. B., Kuckertz, J. M., Siegle, G. J., Ladouceur, C. D., Silk, J. S., Ryan, N. D., … Amir, N. (2015). Empirical recommendations for improving the stability of the dot-probe task in clinical research. Psychological Assessment, 27(2), 365-376. doi:https://doi.org/10.1037/pas0000036
Rêgo, G. G., Lapenta, O. M., Marques, L. M., Costa, T. L., Leite, J., Carvalho, S., … Boggio, P. S. (2015). Hemispheric dorsolateral prefrontal cortex lateralization in the regulation of empathy for pain. Neuroscience Letters, 594, 12-16. https://doi.org/10.1016/j.neulet.2015.03.042
Ripper, C. A., Boyes, M. E., Clarke, P. J. F., & Hasking, P. A. (2018). Emotional reactivity, intensity, and perseveration: Independent dimensions of trait affect and associations with depression, anxiety, and stress symptoms. Personality and Individual Differences, 121, 93-99. https://doi.org/10.1016/j.paid.2017.09.032
Sanchez-Lopez, A., Vanderhasselt, M.-A., Allaert, J., Baeken, C., & De Raedt, R. (2018). Neurocognitive mechanisms behind emotional attention: Inverse effects of anodal tDCS over the left and right DLPFC on gaze disengagement from emotional faces. Cognitive, Affective, and Behavioral Neuroscience, 18(3), 485-494. doi:https://doi.org/10.3758/s13415-018-0582-8
Smits, F. M., Schutter, D. J. L. G., van Honk, J., & Geuze, E. (2020). Does non-invasive brain stimulation modulate emotional stress reactivity? Social Cognitive and Affective Neuroscience, 15(1), 23-51. doi:https://doi.org/10.1093/scan/nsaa011
Tatemoto, T., Yamaguchi, T., Otaka, Y., Kondo, K., & Tanaka, S. (2013). Anodal Transcranial Direct Current Stimulation over the Lower Limb Motor Cortex Increases the Cortical Excitability with Extracephalic Reference Electrodes, Berlin, Heidelberg.
Vicario, C. M., Salehinejad, M. A., Felmingham, K., Martino, G., & Nitsche, M. A. (2019). A systematic review on the therapeutic effectiveness of non-invasive brain stimulation for the treatment of anxiety disorders. Neuroscience & Biobehavioral Reviews, 96, 219-231. doi:https://doi.org/10.1016/j.neubiorev.2018.12.012
Vierheilig, N., Mühlberger, A., Polak, T., & Herrmann, M. J. (2016). Transcranial direct current stimulation of the prefrontal cortex increases attention to visual target stimuli. Journal of neural transmission, 123(10), 1195-1203. doi:https://doi.org/10.1007/s00702-016-1542-5
Voss, M., Ehring, T., & Wolkenstein, L. (2019). Does Transcranial Direct Current Stimulation Affect Post-stressor Intrusive Memories and Rumination? An Experimental Analogue Study. Cognitive Therapy and Research, 43(3), 535-549. doi:https://doi.org/10.1007/s10608-018-9976-8
Watson, D., Clark, L. A., & Tellegen, A. (1988). Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology, 54(6), 1063-1070. doi:https://doi.org/10.1037/0022-3514.54.6.1063
Williams, J. M. G., Watts, F. N., MacLeod, C., & Mathews, A. (1997). Cognitive psychology and emotional disorders (2nd). Oxford, England: John Wiley & Sons.
Wilson, E., & MacLeod, C. (2003). Contrasting two accounts of anxiety-linked attentional bias: Selective attention to varying levels of stimulus threat intensity. Journal of Abnormal Psychology, 112(2), 212-218. doi:https://doi.org/10.1037/0021-843X.112.2.212
Zvielli, A., Bernstein, A., & Koster, E. H. (2015). Temporal dynamics of attentional bias. Clinical Psychological Science, 3(5), 772-788. https://doi.org/10.1177/2167702614551572
Acknowledgements
The authors acknowledge the contribution of Kaitlin Wilson in testing a proportion of participants associated with this study.
Open practices statement
The data and materials for the experiment reported here are available via contact to the corresponding author. The experiment reported in the current study was not preregistered.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Clarke, P.J.F., Van Bockstaele, B., Marinovic, W. et al. The effects of left DLPFC tDCS on emotion regulation, biased attention, and emotional reactivity to negative content. Cogn Affect Behav Neurosci 20, 1323–1335 (2020). https://doi.org/10.3758/s13415-020-00840-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13415-020-00840-2