Abstract
When dissimilar monocular images are viewed simultaneously by the two eyes, stable binocular vision gives way to unstable vision characterized by alternations in dominance between the two images in a phenomenon called binocular rivalry. These alternations in perception reveal the existence of inhibitory interactions between neural representations associated with conflicting visual inputs. Binocular rivalry has been studied since the days of Wheatstone, but one recent strategy is to investigate its susceptibility to influences caused by one’s own motor activity. This paper focused on the activity of walking, which produces an expected, characteristic direction of optic flow dependent upon the direction of one’s walking. In a set of experiments, we employed virtual reality technology to present dichoptic stimuli to observers who walked forward, backward, or were sitting. Optic flow was presented to a given eye, and was sometimes congruent with the direction of walking, sometimes incongruent, and sometimes random, except when the participant was sitting. Our results indicate that, while walking had a reliable influence on rivalry dynamics, the predominance of congruent or incongruent motion did not.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
When the two eyes view different objects or scenes at the same time, stable binocular single vision gives way to unstable visual competition, with the two views achieving perceptual dominance alternately over time (Wheatstone, 1838). Called binocular rivalry, this beguiling phenomenon discloses the existence of inhibitory interactions between neural representations associated with conflicting visual inputs (Blake & Logothetis, 2002; Tong et al., 2006). One strategy for learning details about this neural competition is to ascertain what factors influence the relative predominance and the dynamics of rivalry (i.e., the pattern of alternations in dominance over time), and in recent years this strategy has led to some intriguing findings. We have learned, for example, that rivalry is susceptible to top-down influences such as expectations based on prior experience (Anderson et al., 2011), scene-based congruence (Mudrik et al., 2011), visual imagery (Pearson et al., 2008), symbolic magnitude (Paffen et al., 2011), and attention (Neisser & Becklen, 1975; Lack, 1978; Ooi & He, 1999; see also reviews by Paffen & Alais, 2011, and Dieter & Tadin, 2011). As well, we have learned in recent years that binocular rivalry dynamics are influenced by information conveyed by non-visual sensory modalities, such as hearing (e.g., Conrad et al., 2010; Lunghi et al., 2014), touch (e.g., Lunghi et al., 2010) and olfaction (e.g., Zhou et al., 2010). These results, too, are reshaping our thinking about the bases of neural competition underlying binocular rivalry (Blake, 2014).
Remarkably, even one’s own hand movements can impact binocular rivalry dynamics when those hand movements control the direction of rotation about the vertical axis of one of the two rival stimuli (Maruya et al. 2007). That finding led us to wonder whether motor influences on rivalry would extend to a more large-scale activity—walking—that produces characteristic patterns of optic flow depending on one’s direction of walking. For our study we used virtual reality technology to present dichoptic (i.e., rival) motion flow fields separately to the two eyes of an observer who tracked fluctuations in rivalry while walking forward, walking backward, or sitting. The optic flow presented to a given eye was either congruent or incongruent with the direction of walking, except, of course, when the participant was sitting. Our question was whether a given direction of walking would bias rivalry in favor of the flow-field coinciding with that activity. Ours is not the first study to employ complex optic flow patterns as rival stimuli, but it is the first to do so in the context of self-motion. In several earlier studies using rival targets simulating complex motion patterns, it was found that visual motion simulating expansion enjoyed a bias during rivalry over dots simulating contraction, although there was some disagreement among these studies about the specific dependence of that bias on the form of the motion stimuli (Parker & Alais, 2007; Malek et al., 2012; Conrad et al., 2013; Holten et al., 2015). Our aim, however, was to learn whether the predominance of expansion and contraction was dependent on one’s walking direction. As we document in this paper—and to our surprise—it did not, although we did discover that walking per se had a reliable influence on rivalry dynamics.
General methods
Each of the experiments presented in this paper utilized college-age students aged 18–25 years as participants, all recruited from Vanderbilt and other nearby universities.Footnote 1 Participants were required to have either 20/20 or corrected to 20/20 vision to be eligible. Participants were compensated at a rate of US $10/hour, and each experiment lasted approximately 1 h. Participants were naïve about the purpose of the experiment, until they were debriefed at the conclusion of the test session. None had knowingly experienced binocular rivalry prior to participating in an experiment, so before each test session participants were shown the alternative rival stimuli they should expect to see (Fig. 1), and they were given instructions on how to respond with button presses when they experienced exclusive dominance of each of those rival alternatives. The instructions also stressed the possibility of experiencing mixed dominance (i.e., portions of both rival stimuli visible simultaneously), and they were told to make no button responses when experiencing mixed dominance. Participants received 12 training trials during Experiment 1 only; in all other experiments, participants began immediately as Experiment 1 demonstrated that no training was required.
Schematics of rival stimuli used in the three experiments. For random-dot motion stimuli (shown as dot vectors), individual dots moved outward from the center (expansion, EXP), inward toward the center (contraction, CON) or in random (RAN) directions, with all dots moving at fixed speeds governed by participants’ walking speeds. In Experiment 1, dots viewed by one eye were lighter than the background, and dots viewed by the other eye were darker than the background. Experiment 3 also included stationary, orthogonally oriented gratings as rival stimuli. In all experiments, stimulus types were counterbalanced between eyes over trials
During each trial, participants wore an NVIS (Reston, VA) nVisor SX-60 head mounted display (HMD). The SX-60 is a reflective liquid crystal on silicon (LCOS) type display that permits independent stimulation of each eye (dichoptic viewing of moving stimuli of the sort shown schematically in Fig. 1), and it can generate a full range of 8-bit RGB colors with resolution of 1280 x 1024 for each eye’s display. The optics in the HMD were focused at infinity. The rendering and software system used to drive the HMD was Vizard (Worldviz, Santa Barbara, CA). The graphics cards performing the rendering were either an Nvidia Quadro FX 3800 or GeForce 8800 GTX. Linear 8-bit color values computed by the rendering system were gamma corrected internally by the system using a standard LCD (γ = 2.2) monitor value. On each trial, participants walked forward, walked backward, or sat in a chair for the duration of the trial. For trials involving walking, the participant was guided along a circular pathway by gripping an apparatus and walking. This apparatus is depicted in Fig. 2, and the resulting circular pathway was 22 ft in circumference. Participants were instructed to walk at a comfortable, constant pace during each trial; if necessary, participants were encouraged to adjust walking speed if they exhibited brief pauses between steps (i.e., temporary slowing), or if they were unable to maintain constant curvature as indicated by the distance between their bodies and the grip apparatus they were holding (i.e., speeding up). In fact, such encouragement was infrequent, and participants were able to maintain the desired walking speed of 132 ft/min, meaning six revolutions of the circular path per 60-s trial. Participants did not need to reverse direction during a trial, owing to the tethering of the cable. Walking direction was reversed between trials, such that on even-numbered walking trials participants walked in one direction, and in odd-numbered trials they walked in the other direction. This means that a given person’s facing direction did not change during an experiment, and each person was allowed to chose a preferred facing direction. In addition, the participant’s walking speed was constantly monitored and recorded using a WorldViz (Santa Barbara, CA) Precise Position Tracking (PPT) system comprising four cameras that tracked the position of two LEDs attached to the HMD.
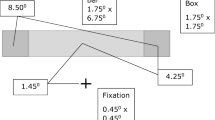
Apparatus used in all experiments. Participants wore a virtual reality head-mounted display (HMD) that afforded dichoptic stimulation, and, on some trials they walked at a natural pace around a large, circular path, walking either forward or backward for the duration of a given trial, and pressing keys on a response box to indicate rivalry dominance. The control pack interfacing the display and response box to the computer was worn by an experimenter who trailed the participant while walking. No participant complained about the task or expressed discomfort walking forward or backward in this manner
The participants’ task was to signify which one of two rival stimuli was exclusively visible (i.e., dominant) by pressing one of two buttons on a Wiimote motion controller attached to the hand-grip held by the participant while walking. Durations of successive button presses were recorded throughout the trial. During each trial involving walking, the speed of the motion tokens defining a rival stimulus was matched to the current average speed of the participant (computed over a 10-s, sliding window), the intention being to give the impression that the participants walking was linked to the motion vector associated with one of the two rival stimuli. For conditions when the participant was sitting, the speeds of the moving stimuli were matched to the average walking speed of that individual (computed over the two previous walking trials). Each trial lasted at least 60 s, and was extended to include the entire final duration of dominance (i.e., if a button was being pressed when the 60 s time elapsed, the trial continued until that button was released, thus precluding truncation of the last dominance duration). As mentioned earlier, no button presses were to be made when experiencing mixed dominance.
Overview of experiments
The overarching goal of this project was to learn whether rivalry between motion signifying expansion and motion signifying contraction was influenced by the walking direction—forward vs backward—of the person viewing that rivalry. Each of the following experiments employed rival motions designed to test a specific hypothesis, and the particulars of those stimuli are described in the results section for each specific experiment. In all experiments, each eye’s circular rival target subtended 6.9° visual angle—a size selected so that the optic flow animations could accommodate a large number of moving dots that would extend into the near periphery, and, therefore, provide a compelling sense of expansion and contraction. It is worth noting that these displays, when viewed while standing still, did not induce an illusory sense of self-motion (i.e., vection), which is not surprising given that vection is typically associated with motion over large regions of the visual field.
The average luminance values of the rival stimuli were 23 cd/m2 for the left eye LCOS display, and 18 cd/m2 for the right eye LCOS display. These differences in light levels between the two LCOS displays were caused by unanticipated differences between the two LCOS displays, and they went unnoticed by us. It was only when measuring the average luminance values after completion of the study that the disparity was discovered. Fortunately, all stimulus conditions were counterbalanced between the eyes, thus the difference in mean luminance was not a contributing factor in our study. Moreover, the luminance values of the stimuli themselves were also subject to this difference between the LCOS displays, so the luminance contrasts of our stimuli were equivalent for the two eyes. In all conditions except one, random-dot cinematograms (RDCs) were used to portray rival motion, the one exception being a condition in Experiment 3 that utilized orthogonally oriented gabor patches. For the RDC stimuli, each eye’s view contained 100 dots, and their luminance level was always 0.3 log-units different from the mean luminance background. The circular rival targets viewed dichoptically appeared against a black background (unmeasurably low luminance) such that the circular shape of each of two targets was highly conspicuous and afforded strong fusion locks for the two eyes’ views.
On each trial conducted throughout all three experiments, the computer controlling the stimuli logged every instance of a button press by the participant. This data record was processed off-line into individual instances of rivalry dominance, defined as a button press followed by a button release. Periods during which neither button was being pressed defined a mixed dominance period, i.e., a period of time in which the participant saw bits and pieces of both stimuli. Over all three experiments, the incidence of mixtures was 10% of the total viewing period averaged across participants and conditions—a value not surprising given the relatively large size of the rival stimuli. Statistical analyses focused on average dominance durations for specific conditions and on cumulative frequency histograms for all durations associated with a given condition. The histograms, probability density functions, and cumulative distribution functions were created from normalized durations formed by dividing each event for a given participant by that person’s average duration computed from all rivalry dominance events (but not mixtures) experienced by that person.
Experiment 1: expansion vs contraction with colored dots
Methods
In this first experiment each of 24 students (13 females) participated in a single, 70-min test session consisting of 48 trials each lasting approximately 60 s. On each trial, rival stimuli comprised moving dots (RDCs), with all dots viewed by one eye being 0.3 log-units lighter than the mean luminance background against which they appeared, and all dots viewed by the other eye being 0.3 log-units darker than the mean luminance background against which they appeared (Fig. 1 upper panel). On two-thirds of the trials, dots viewed by the two eyes were also distinguished by their radial motion vectors: on 32 trials one eye’s 100 dots all moved radially outward from the center of the circular dot array simulating expansion (EXP), and the other eye’s dots all moved radially inward toward the center of the array simulating contraction (CON). All of the EXP dots, and all of the CON dots, moved at a uniform speed that was governed by the walking speed of the participant. On the remaining 16 trials, the dots in both eyes’ views moved in random directions throughout the trial duration (RAN), at the same uniform speed as EXP and CON dot motions. The left- and right-eye dot arrays in the RAN condition were distinguishable by their relative gray levels only. We included this RAN condition to allow us to assess effects on rivalry of structured optic flow compared to unstructured random motion.
The participant’s task was to track rivalry based on the color (i.e., light gray vs dark gray) of the RDC dots comprising the currently dominant motion array. In training trials, and during actual test trials, one eye viewed a RDC portraying EXP motion while the other eye viewed a RDC portraying CON motion, or both eyes viewed RDCs displaying RAN motion that was uncorrelated between the two eyes’ views (but were distinguishable based on color). These combinations produced three conditions: EXP left-eye/CON right-eye, CON left-eye/EXP right-eye, or RAN left-eye/RAN right-eye. Crossing these three conditions with two values of color (black dots seen by one eye and white dots by the other) yielded six stimulus conditions in total. During Experiment 1, participants received these 6 conditions crossed with two walking directions (forward or backward), yielding 12 conditions repeated four times for 48 trials. The direction of walking was alternated over trials, but the first walking direction, and the other conditions, were randomized. Prior to the actual experiment, each participant was familiarized with the stimuli and the tracking task by tracking rivalry between EXP/CON and between RAN/RAN while sitting. For this initial familiarization phase, dot motion speed was matched to the walking speed measured while the first author circumnavigated the walking path (since this phase preceded any walking conditions by the participant). Data from the familiarization stage were not used in the analyses of the results.
Results
This experiment produced almost 29,000 dominance duration values (i.e., button presses) combined over the 24 participants and 48 trials, each of which lasted at least 60 s. Among those button press durations were some that were too brief to realistically qualify as plausible dominance states. We decided, therefore, to eliminate any button press lasting less than one-eighth of a second, a cutoff that culled 1.95% of the data from the total set of durations. These ultra-brief button presses were distributed equally between the two walking conditions. There were also a very small number of button presses each lasting 30 s or more, unusually large values for rivalry durations. But, in the absence of some principled reason for treating them as aberrant button presses, we elected to leave them in the data set. As explained later in this section, removal of durations longer than an arbitrarily chosen, long value did not change the pattern of results, which is not surprising given paucity of these ultra-long duration values.
Figure 3 shows the individual dominance duration histograms for each of the 24 participants who participated in this experiment; these histograms comprise aggregated dominance durations for RAN, EXP and CON stimuli. The distributions are all unimodal and skewed toward longer durations, canonical characteristics of duration histograms for binocular rivalry and other forms of bistable perception (Levelt, 1965; Fox & Herrmann, 1967; Lehky, 1995; Brascamp et al., 2005). Moreover, these distributions also reveal substantial differences among participants in terms of the peak locations along the duration axis, implying that individuals vary in terms of their alternation rates. This feature of our results is also seen in other studies of binocular rivalry (Carter & Pettigrew, 2003; Miller et al., 2010; Hancock et al., 2012; Patel et al., 2015). We conclude, therefore, that the act of walking has not changed binocular rivalry in any fundamental way.
Frequency histograms of dominance durations for each of the 24 participants in Experiment 1
We also computed an index of eye dominance for each participant in the following way. For each of the 48 trials, we summed the dominance durations for each eye separately, and expressed each of those two sums as a percentage of the total trial duration (which was ~60 s). We then subtracted one percentage from the other to derive an index of eye dominance on that given trial, and we repeated this procedure for each of the 48 trials. We computed the average of those 48 difference values to derive an overall eye dominance ‘score’ for that person. This was done for each participant, and the 24 resulting values of that eye dominance index (i.e., one index for each person) showed a majority of values favoring left-eye dominance, with the median difference value being 5% greater for left-eye dominance. These individual differences in eye dominance cannot be attributed to direction of walking, or to differences in effectiveness of EXP/CON/RAN displays, because those factors were counterbalanced between eyes. We surmise that this slight bias toward left-eye dominance might be related to the disparity in mean luminance between left- and right-eye LCOS displays, discovered after the fact. Three earlier rivalry studies reporting eye dominance results did not observe this trend toward left-eye dominance (Ooi & He, 2001; Al-Dossari et al., 2015; Zheleznyak et al., 2015).
To pool results over participants despite the individual differences seen in Fig. 3, we normalized the data as described above (i.e., dividing each dominance duration for an individual by that person’s overall mean duration). The histogram in Fig. 4a shows the distribution of normalized durations for all conditions and all participants. This histogram can also be plotted as a cumulative distribution function, i.e., CDF (Fig. 4b). When the data comprising this CDF is divided into two sets, one associated with walking forward, and the other associated with walking backward, we see that the resulting pair of CDFs is indistinguishable visibly (Fig. 4c), and they are indistinguishable statistically when compared using a two-parameter Kolmogorov-Smirnov test (conditions μforward = 2.02/μbackward = 2.03; KS p = 0.47). In other words, direction of walking per se did not affect rivalry dynamics. Histograms in Fig. 4d plot the mean of each individual’s normalized dominance durations separated into walking forward and walking backward, as well as the standard error of the mean.
a Normalized dominance durations pooled over all observers and conditions. b Cumulative distribution function (CDF) derived from the histogram in a. c CDFs for dominance durations measured while participants walked forward (red curve) and while they walked backward (blue curve). d Mean normalized dominance durations. Error bars Standard error
We also divided the entire normalized data set into three sets defined by dominance durations associated with the three RDC motion types—EXP, CON and RAN—without regard to direction of walking. Pairwise comparisons of the CDFs associated with those three data sets were then performed using the KS test (Fig. 5a–c). Both EXP and CON dominance duration distributions were significantly different than the dominance duration distribution associated with RAN (EXP/RAN P = .012; CON/RAN P = .004), but the dominance duration distribution for EXP was no different from the distribution for CON (P = .279). Inspection of the normalized duration histograms in Fig. 5d confirms that RAN durations were slightly longer than EXP and CON durations. An earlier study of rivalry between dissimilar, structured motion viewed by stationary observers did not include RAN among their stimuli, so we cannot meaningfully compare our results to theirs (Malek et al., 2012).
a–c Pairwise comparisons of the CDF distributions for the normalized dominance durations measured in Experiment 1 EXP, CON and RAN. d Mean normalized dominance durations and standard error
Turning to the main question of interest, does the tendency for a given direction of motion to be dominant in rivalry depend on the direction in which the participant was walking? To answer this question, we analyzed the normalized dominance durations separately for EXP and for CON as a function of the direction of walking, i.e., combinations that produced a data set comprised of durations from the two congruent conditions (EXP dominant when walking forward and CON dominant when walking backward), and a data set comprised of durations from the two incongruent conditions (EXP dominant when walking backward and CON dominant when walking forward). Figure 6a shows the CDFs for those two conditions, congruent and incongruent. Because these durations were normalized to the same grand means, differences between congruent and incongruent conditions would show up as non-overlapping in portions of those CDFs, but that is not visibly evident. Confirming what those curves imply, results from a paired t-test of the subject means for congruent and incongruent trials (Table 1) reveal no significant differences between dominance durations for those two conditions. That conclusion is further substantiated by the non-significant results from a two-parameter Kolmogorov-Smirnov test of the equality of continuous, one-dimensional probability distributions (also summarized in Table 1). Also included in Table 1, and subsequent Tables 2, 3 and S1, are the means and standard deviations of the actual duration values (i.e., not normalized), to provide readers with a sense of the actual duration values associated with those conditions. To reiterate, for purposes of statistical analyses, we normalized the data so as to remove individual differences from the sources of variance in the group data.
CDF distributions of the normalized durations of Experiment 1 for congruent and incongruent conditions (a), congruent and incongruent conditions after long duration events were pruned (b), and for the two color conditions (c). Mean normalized dominance durations and standard error bars (d–f) corresponding to the same data in (a–c) respectively
We wondered whether this failure to uncover an effect of congruence might arise from differences in the relative strengths of the different colored dots defining EXP and CON (recall that color was randomized across conditions, so it is conceivable that an effect of congruence could be masked by a general difference in the efficacy of light vs dark dots). This conjecture, however, does not receive support when we compare the CDFs for the two colors: the two conditions were indistinguishable (Fig. 6b), and the paired t-tests confirm that conclusion (Table 1).
As mentioned earlier, this large data set included some very long dominance durations. To insure that those values were not masking a subtle but significant difference between the congruent and incongruent conditions, we repeated our analyses on this same data set following removal of duration values greater than 3 standard deviations above the median of the log-normal distribution (Brascamp et al., 2005)—which cut-off eliminated all durations greater than 15.92 s that constituted 0.3% of the original data set. The CDFs for congruent and incongruent conditions created from this pruned data set were again indistinguishable both visually (Fig. 6c) and statistically (Table 1).
The failure to find an effect of congruence on rivalry dynamics between EXP and CON stimuli led us to wonder whether the instructions for the tracking task itself might be responsible for this negative result. Participants tracked dominance states based on the color of the dots (i.e., light gray vs dark gray) defining the dominant motion array; they were not instructed explicitly to track rivalry based on the pattern of motion. We had them track color so that we could use the same instructions for the RAN condition, where pattern of motion would have been useless as a signature of dominance. From other work, however, it is known that rivalry between dissimilar targets defined by multiple different stimulus dimensions (e.g., form and color) can include periods where a stimulus feature imaged in one eye dominates simultaneously with a second feature imaged in the other eye, producing a hybrid, albeit illusory, state of dominance (Creed, 1935; Carny et al., 1987; Hong & Blake, 2009; Holmes et al., 2006). Perhaps, then, by having our participants track color, not motion, we unwittingly obscured an effect of motion rivalry because of contamination by color rivalry. To address this possibility we performed Experiment 2.
Experiment 2: Is color responsible for the absence of a congruence effect?
To rule out color as a source of rivalry in our motion stimuli, we repeated the task used in Experiment 1, i.e., tracking rivalry while walking. This time, however, participants were explicitly instructed to report exclusive dominance of EXP and CON, both of which were portrayed by equivalently colored dots (i.e., all light gray, 0.3 log-units lighter than the background—middle panel, Fig. 1). For this second experiment, we excluded the RAN condition (it was impossible to discern stimulus dominance when both eyes viewed RAN motion of identically colored dots). We also added a condition where the observer did not walk but instead sat while tracking rivalry, our aims being to learn whether walking alone might have an influence on rivalry dynamics and, also, to allow participants to rest periodically during the test session. This experiment produced 4100 dominance durations.
Methods
Twelve individuals participated in this experiment, which comprised a total of 30 trials. On each trial, one eye viewed EXP and the other viewed CON, and over trials those two rival directions were counterbalanced between left- and right-eyes. On each trial, the participant walked forward, walked backward, or sat while tracking rivalry between EXP and CON. In this experiment, all dots, whether EXP or CON, were the same color, i.e., light gray against a darker gray background; participants were instructed to track rivalry based on exclusive visibility of structured optic flow direction, i.e., EXP vs CON. Prior to formal testing, observers were shown examples of these two optic flow patterns, and it was stressed that they were not to press a key when experiencing mixtures of the patterns of dot motion. The order of trials followed the sequence “walking forward, sitting, walking backward,” and this was repeated ten times for a total of 30 trials. The assignment of motion type to eyes was haphazard over trials with the stipulation that this factor was balanced over the entire set of trials for the three activity conditions.
Results
Figure 7a presents the overlaid CDFs for the congruent conditions (EXP while walking forward, and CON while walking backward) and the incongruent conditions (EXP while walking backward, and CON while walking forward). Once again, we see essentially no difference between the two sets of dominance durations, and this impression is substantiated by the non-significant P value produced by the K-S test, shown in Table 2. To our surprise, however, differences in dominance durations are evident between the results for sitting vs walking (Fig. 7b) and those differences are highly significant as revealed by the K-S (Table 2): dominance durations for these optic flow patterns generally tend to last longer when one is walking than when one is sitting.
Pairwise comparisons of CDFs of the normalized dominance durations measured in Experiment 2 for congruent and incongruent conditions (a) and for walking and sitting conditions (b). Mean normalized dominance durations and standard error bars for congruent and incongruent conditions (c) and for walking and sitting conditions (d)
This unexpected finding led us to wonder whether walking while tracking rivalry might constitute a dual-task situation that slows a participant’s reaction times to spontaneous transitions in rivalry state. To test this conjecture, we performed a third experiment that incorporated not only RDC rivalry but also a new pair of rival stimuli that did not portray motion. Those stimuli comprised orthogonally oriented, stationary gabor patches (±45° from vertical) that generated robust rivalry but portrayed no sense of optic flow. If walking and tracking rivalry indeed constitute a dual-task challenge that slows a participant’s reaction times to dominance state changes, we would expect to find the same pattern of differences between dominance durations for gratings measured during walking and during sitting.
Experiment 3: Walking vs sitting/moving vs stationary rival stimuli
Methods
In this experiment, 12 individuals participated in a testing session that lasted approximately 1 h. A total of 32 rivalry tracking trials were administered, with half of those trials devoted to rivalry between EXP and CON (both portrayed using light gray dots against a darker background), and the other half of the trials devoted to rivalry between the stationary, orthogonally oriented gabor patches (Fig. 1c), both of spatial frequency 0.7 c/° and 50% contrast). The order of rivalry stimuli over trials was randomized, and the eye receiving a given motion type or a given gabor patch orientation was counterbalanced over trials. For this experiment, we devoted an equal number of trials to sitting and to walking, so as to equate the two conditions of interest in terms of number of trials. So, to summarize, the 32 trials administered to each participant consisted of 16 trials of rivalry between EXP/CON, and 16 trials of rivalry between gabors oriented +45°/–45°; for both categories of rival stimuli, 8 of the trials were performed while sitting, 4 of the trials were performed while walking forward, and 4 of the trials were performed while walking backward. Walking and sitting were alternated over trials. This experiment produced roughly 3600 and 4600 dominance duration between the motion type stimuli and the gabor patch stimuli, respectively.
Results
Figure 8a and Table 3 show once again that direction of walking has no influence on rivalry dynamics associated with dichoptic viewing of EXP and CON; this replicates the negative effect of congruency found in our previous three experiments. Moreover, we once again see that dominance durations of EXP and CON tend to be longer when the participant is walking compared to sitting (Fig. 8b and Table 3). Importantly, however, we find no evidence of an influence of walking vs sitting on the dominance durations associated with rivalry between the stationary gabor patches (Fig. 8c and Table 3). Thus, we are disinclined to conclude that dual-task demands underlie the walking/sitting difference obtained with optic flow rival stimuli.
Pairwise comparisons of CDFs of the normalized dominance durations measured in Experiment 3 for congruent and incongruent conditions (a), walking and sitting conditions with moving rival stimuli (b), and walking and sitting conditions with stationary gabor patches (c). Mean normalized dominance durations and standard error bars for congruent and incongruent conditions (d), walking and sitting conditions with moving rival stimuli (e), and walking and sitting conditions with stationary gabor patches (f)
Discussion
Decades ago, binocular rivalry was considered by many to be a bottom-up driven visual phenomenon whose dynamics were determined primarily by the interplay of adaptation and inhibition within early visual cortex (Levelt, 1965; Matsuoka, 1984; Mueller, 1990; Lehky & Blake, 1991; Sengpiel et al., 1995). But that view began to change as evidence mounted for the involvement influences originating beyond early visual cortex, influences including attentional modulation of dominance at the onset of rivalry (Mitchell et al., 2004) and interocular grouping in the formation of global, coherent dominance during rivalry (e.g., Kovács et al., 1996). Adding fuel to that revision in thinking about rivalry were emerging results from neurophysiology pointing to a hierarchy of brain areas involved in the resolution of rivalry (Sengpiel, 1997; Leopold & Logothetis, 1999; Blake & Logothetis, 2002). There then followed a stream of papers showing all sorts of intriguing “top-down” influences on the spatial and temporal properties of binocular rivalry (reviewed by Blake, 2014), adding further weight to this revised view of rivalry. Given this momentum in thinking about rivalry—and the growing appreciation that action can influence visual perception in other contexts (e.g., Witt, 2011)—we were genuinely surprised to find that rivalry between competing dichoptic patterns of optic flow is uninfluenced by the direction in which an observer is walking while experiencing binocular rivalry between those conflicting flow patterns. In the following paragraphs, we consider various alternative explanations for this failure to find an effect of action on perception.
First, one might question whether EXP and CON portrayed by RDCs are sufficient to trigger binocular rivalry, because the small, individual dots imaged in left- and right-eyes are themselves not continuously falling on corresponding retinal areas. Perhaps, then, EXP and CON dots might tend to fuse into a hybrid pattern that masks any sense of the two distinct monocular flow fields, something akin to what has been reported for rivalry between dissimilarly oriented moving contours (Andrews & Blakemore, 2002). In fact, however, rivalry between EXP and CON strongly resembles other forms of rivalry, including conventional contour-based rivalry: the successive periods of dominance are clear-cut, they last for durations comparable to those reported for other forms of rivalry (recall Fig. 3a), and they are accompanied by intervening periods of mixtures. We would note, too, that other studies of motion-induced rivalry have successfully used RDCs of the sort we employed (Blake et al., 1985, 1998; Chen et al., 2001; Parker & Alais, 2007; Malek et al., 2012; Holten et al., 2015). We are thus satisfied that the stimuli we employed are sufficient to generate robust binocular rivalry. Moreover, in a supplemental experiment (Supplement 1), we replicated the main results using, instead of dots, textured wall and ceiling surfaces to present dissimilar perspective-defined optic flow patterns to the two eyes.
A skeptic could also argue that our participants realized that the rival stimuli they were experiencing did not correspond to the actual configuration of the room in which they were being tested. Perhaps, the argument continues, this realization broke the functional connection between one’s walking direction and the perceived optic flow direction one experienced, even though we carefully matched dot speed to each participant’s actual walking speed. We would simply note that many other published studies have reported significant top-down influences on rivalry utilizing test conditions that were less realistic than those we devised. Here are three examples. First, a person with a tube inserted into one nostril is more likely to perceive a given line drawing of an object (e.g., a rose) presented on a video monitor as dominant in rivalry when the inhaled odor being delivered through the tube (e.g., floral) is congruent with the visual object (Zhou et al., 2010). Second, an AM- and FM-modulated tone heard over headphones biases rivalry when conflicting looming and receding motion displays are viewed dichoptically on a pair of video monitors (Conrad et al., 2013). And third, a rotating rival target generated on a video monitor predominates more during rivalry when an observer simultaneously moves a hand-held mouse back and forth in rhythm with the pattern of rotation of the visual stimulus (Maruya et al., 2007). These examples of successful multisensory and motor interactions on binocular rivalry underscore that a participant need not be tricked into believing that what is being seen during rivalry is integrally tied to what one is otherwise experiencing.
Nonetheless, we recognize that there are limitations to the stimuli used in our experiments compared to the optic flow experienced during unconstrained walking in natural environments. Our participants were walking in a circle, and the radius of the circle was within their biomechanical threshold of detection (Steinicke et al., 2010). The optic flow patterns they viewed, however, comprised linear motions, meaning the visual stimuli did not perfectly match the biomechanical concomitants of walking. It is also possible that the tether and cabling for the HMD represented a distraction, although none of the participants complained about this. Still, it would be interesting to know what would happen in an experiment using more naturalistic conditions, perhaps using a wireless HMD in a large tracked space (such a basketball court or indoor track) where long periods of linear walking could be supported. Although not available at the time of this work, commodity level wireless HMDs such as the Samsung Gear VR could be used for this type of stimulus. At the same time, large-field visual simulations could complicate participants’ reports of rivalry, owing to the inevitable piecemeal nature of rivalry with larger rival stimuli (Blake et al., 1992). In this regard, we note that the contrast and field of view in our experiment were carefully tailored to minimize piecemeal rivalry.
Finally, one could question whether our experiments had sufficient power (Cohen, 1992) to reveal an effect of congruency. In response to this concern, we would point out that our three experiments, involving a total of 48 participants, whose results generated 40,546 dominance durations, all point to the same null result. Our decision about sample size was guided by previous studies that have found significant effects of stimulus factors on rivalry dynamics using sample sizes ranging from 12 to 24. Moreover, we did find significant differences between several conditions in our experiments: EXP yielded a significantly different dominance duration distribution than RAN, CON yielded a significantly different dominance duration distribution than RAN, and dominance durations during walking produced significantly longer durations than those found during sitting. Those findings give us confidence that our study was not underpowered.
Turning to other aspects of our findings, we did not find a reliable tendency for EXP to enjoy longer dominance durations than CON—a result consistent with one other study (Parker & Alais, 2007), but inconsistent with another (Conrad et al., 2013). The authors of the study that did find EXP > CON durations opined that this bias for visual looming could arise from the association of looming with potential danger or collision. In other words, radial looming on the retina is attributed to impending collision of moving object approaching a stationary observer, a highly salient, potentially threatening circumstance that commands attention and heightens arousal. The optic flow experienced in our study, however, has a plausible, alterative attribution: it arises from self-produced action without any obvious threat. This realization led us to analyze EXP and CON dominance durations measured during the sitting condition in Experiments 2 and 3, where one’s own actions do not imply self-production of optic flow on the retina. In that combined data set, EXP dominance durations were significantly longer than were CON durations (3.72 vs 3.175 s, T-test P = .003, ks-test P = .00006), mirroring earlier findings (Conrad et al., 2013; Malek et al., 2012; but see Parker & Alais, 2007). So, perhaps walking vetoes the sense of looming and pending collision signified by centrifugal radial motion viewed by a stationary observer.
This conjecture may also shed some light on why walking produces longer dominance durations for EXP and CON than does sitting. Specifically, consider the main effect that sitting vs walking had on dominance durations for optic flow rivalry but not for stationary gabor patch rivalry (Experiment 3). Perhaps this reflects a coarser form of action/perception link than we originally envisioned. Here’s what we have in mind. As one walks about within the environment, retinal optic flow is the natural visual consequence: the association of the two is forged by a lifetime of visual experience during activity. Sitting, however, is not usually accompanied by motion on the retina except that associated with eye movements and head turning, and those characteristically generate translation retinal motion, not radial motion. So perhaps the lengthening of dominance durations in rivalry while walking compared to sitting stems from the high likelihood of experiencing visual motion when walking compared to when sitting. This account is speculative, of course, but one can imagine ways to pursue it. For example, it could be informative to repeat the conditions of Experiment 3 while observers are passively seated in a wheel-chair being pushed by another individual (but this experiment is beyond the technology available to us). It might also be revealing to assess motion rivalry while the participant drives a virtual vehicle in a driving simulator or, instead, sits passively as the passenger in that vehicle (again, an idea we are not equipped to test).
As things stand now, however, our results do not reveal a selective impact of walking direction on interocular competition between conflicting patterns of optic flow. Evidently, the factors that boost dominance of visual motion while one is walking versus sitting are agnostic about the specific pattern of optic flow in relation to the direction of walking. There exist limits, in other words, on the sophistication of context’s impact on binocular rivalry.
Notes
We tested a total of 48 different participants in the three experiments comprising this project (24 in Experiment 1, and 12 each in Experiments 2 and 3). This sample size is considerably larger than that typically used in binocular rivalry experiments in general, and twice as large as the largest sample size among the four previous experiments documenting an effect of complex motion on binocular rivalry. The specific number of individuals used in our experiments allowed us to completely counterbalance the order of trials taking into account stimuli, order of direction of walking and eye.
References
Al-Dossari, M., Blake, R., Brascamp, J. W., & Freeman, A. W. (2015). Chronic and acute biases in perceptual stabilization. Journal of Vision, 15(4), 1–11.
Anderson, E., Siegel, E. H., Bliss-Moreau, E., & Barrett, L. F. (2011). The visual impact of gossip. Science, 332(6036), 1446–1448.
Andrews, T. J., & Blakemore, C. (2002). Integration of motion information during binocular rivalry. Vision Research, 42(3), 301–309.
Blake, R. (2014). Binocular rivalry updated. In: J.S. Werner & L.M. Chalupa (Eds.), The new visual neurosciences (pp. 827–846). MIT Press.
Blake, R., & Logothetis, N. K. (2002). Visual competition. Nature Reviews Neuroscience, 3(1), 13–21.
Blake, R., O’Shea, R. P., & Mueller, T. J. (1992). Spatial zones of binocular rivalry in central and peripheral vision. Visual Neuroscience, 8, 469–478.
Blake, R., Yu, K., Lokey, M., & Norman, H. (1998). Binocular rivalry and motion perception. Journal of Cognitive Neuroscience, 10(1), 46–60.
Blake, R., Zimba, L., & Williams, D. (1985). Visual motion, binocular correspondence and binocular rivalry. Biological Cybernetics, 52(6), 391–397.
Brascamp, J. W., Van Ee, R., Pestman, W. R., & Van Den Berg, A. V. (2005). Distributions of alternation rates in various forms of bistable perception. Journal of Vision, 5(4), 287–298.
Carny, T., Shadlen, M., & Switkes, E. (1987). Parallel processing of motion and colour information. Nature, 328, 647–649.
Carter, O. L., & Pettigrew, J. D. (2003). A common oscillator for perceptual rivalries? Perception, 32(3), 295–305.
Chen, Y., Matthews, N., & Qian, N. (2001). Motion rivalry impairs motion repulsion. Vision Research, 41(27), 3639–3647.
Cohen, J. (1992). Statistical power analysis. Current Directions in Psychological Science, 1(3), 98–101.
Conrad, V., Bartels, A., Kleiner, M., & Noppeney, U. (2010). Audiovisual interactions in binocular rivalry. Journal of Vision, 10(10), 27.
Conrad, V., Kleiner, M., Bartels, A., O'Brien, J. H., Bülthoff, H. H., & Noppeney, U. (2013). Naturalistic stimulus structure determines the integration of audiovisual looming signals in binocular rivalry. PloS One, 8(8), e70710.
Creed, R. S. (1935). Observations on binocular fusion and rivalry. The Journal of Physiology, 84(4), 381–392.
Dieter, K. C., & Tadin, D. (2011). Understanding attentional modulation of binocular rivalry: A framework based on biased competition. Frontiers in Human Neuroscience, 5, 155.
Fox, R., & Herrmann, J. (1967). Stochastic properties of binocular rivalry alternations. Perception & Psychophysics, 2(9), 432–436.
Hancock, S., Gareze, L., Findlay, J. M., & Andrews, T. J. (2012). Temporal patterns of saccadic eye movements predict individual variation in alternation rate during binocular rivalry. i-Perception, 3(1), 88–96.
Holmes, D. J., Hancock, S., & Andrews, T. J. (2006). Independent binocular integration for form and colour. Vision Research, 46(5), 665–677.
Holten, V., Donker, S. F., Verstraten, F. A. J., & van der Smagt, M. J. (2015). Visual directional anisotropy does not account for the directional anisotropy in postural sway. Perception, 44, 477–489.
Hong, S. W., & Blake, R. (2009). Interocular suppression differentially affects achromatic and chromatic mechanisms. Attention, Perception, & Psychophysics, 71(2), 403–411.
Kovács, I., Papathomas, T. V., Yang, M., & Fehér, Á. (1996). When the brain changes its mind: Interocular grouping during binocular rivalry. Proceedings of the National Academy of Sciences of the United States of America, 93(26), 15508–15511.
Lack, L. C. (1978). Selective attention and the control of binocular rivalry (Vol. 11). Paris: Mouton De Gruyter.
Lehky, S. R. (1995). Binocular rivalry is not chaotic. Proceedings of the Royal Society of London B: Biological Sciences, 259(1354), 71–76.
Lehky, S. R., & Blake, R. (1991). Organization of binocular pathways: Modeling and data related to rivalry. Neural Computation, 3(1), 44–53.
Leopold, D. A., & Logothetis, N. K. (1999). Multistable phenomena: Changing views in perception. Trends in Cognitive Sciences, 3(7), 254–264.
Levelt, W. J. M. (1965). On binocular rivalry. Soesterberg, The Netherlands: Institute for Perception RVO-TNO.
Lunghi, C., Binda, P., & Morrone, M. C. (2010). Touch disambiguates rivalrous perception at early stages of visual analysis. Current Biology, 20(4), R143–R144.
Lunghi, C., Morrone, M. C., & Alais, D. (2014). Auditory and tactile signals combine to influence vision during binocular rivalry. The Journal of Neuroscience, 34(3), 784–792.
Malek, N., Mendoza-Halliday, D., & Martinez-Trujillo, J. (2012). Binocular rivalry of spiral and linear moving random dot patterns in human observers. Journal of Vision, 12(10), 16. doi:10.1167/12.10.16
Maruya, K., Yang, E., & Blake, R. (2007). Voluntary action influences visual competition. Psychological Science, 18(12), 1090–1098.
Matsuoka, K. (1984). The dynamic model of binocular rivalry. Biological Cybernetics, 49(3), 201–208.
Miller, S. M., Hansell, N. K., Ngo, T. T., Liu, G. B., Pettigrew, J. D., Martin, N. G., & Wright, M. J. (2010). Genetic contribution to individual variation in binocular rivalry rate. Proceedings of the National Academy of Sciences of the United States of America, 107(6), 2664–2668.
Mitchell, J. F., Stoner, G. R., & Reynolds, J. H. (2004). Object-based attention determines dominance in binocular rivalry. Nature, 429(6990), 410–413.
Mudrik, L., Breska, A., Lamy, D., & Deouell, L. Y. (2011). Integration without awareness expanding the limits of unconscious processing. Psychological Science, 22(6), 764–770.
Mueller, T. J. (1990). A physiological model of binocular rivalry. Visual Neuroscience, 4(01), 63–73.
Neisser, U., & Becklen, R. (1975). Selective looking: Attending to visually specified events. Cognitive Psychology, 7(4), 480–494.
Ooi, T. L., & He, J. Z. (1999). Binocular rivalry and visual awareness: The role of attention. Perception, 28, 551–574.
Ooi, T. L., & He, Z. J. (2001). Sensory eye dominance. Optometry, 72, 168–178.
Paffen, C. L., & Alais, D. (2011). Attentional modulation of binocular rivalry. Frontiers in Human Neuroscience, 5, 1–10.
Paffen, C. L., Plukaard, S., & Kanai, R. (2011). Symbolic magnitude modulates perceptual strength in binocular rivalry. Cognition, 119(3), 468–475.
Parker, A., & Alais, D. (2007). A bias for looming stimuli to predominate in binocular rivalry. Vision Research, 47(20), 2661–2674.
Patel, V., Stuit, S., & Blake, R. (2015). Individual differences in the temporal dynamics of binocular rivalry and stimulus rivalry. Psychonomic Bulletin & Review, 22(2), 476–482.
Pearson, J., Clifford, C. W., & Tong, F. (2008). The functional impact of mental imagery on conscious perception. Current Biology, 18(13), 982–986.
Sengpiel, F. (1997). Binocular rivalry: Ambiguities resolved. Current Biology, 7(7), R447–R450.
Sengpiel, F., Blakemore, C., & Harrad, R. (1995). Interocular suppression in the primary visual cortex: A possible neural basis of binocular rivalry. Vision Research, 35(2), 179–195.
Steinicke, F., Bruder, G., Jerald, J., Frenz, H., & Lappe, M. (2010). Estimation of detection thresholds for redirected walking techniques. IEEE Transactions on Visualization and Computer Graphics, 16(1), 17–27.
Tong, F., Meng, M., & Blake, R. (2006). Neural bases of binocular rivalry. Trends in Cognitive Sciences, 10(11), 502–511.
Wheatstone, C. (1838). Contributions to the physiology of vision—Part the first. On some remarkable, and hitherto unobserved, phenomena of binocular vision. Philosophical Transactions of the Royal Society of London, 128, 371–394.
Witt, J. K. (2011). Action’s effect on perception. Current Directions in Psychological Science, 20(3), 201–206.
Zheleznyak, L., Alarcon, A., Dieter, K. C., Tadin, D., & Yoon, G. (2015). The role of sensory ocular dominance on through-focus visual performance in monovision presbyopia corrections. Journal of Vision, 15(6), 17.
Zhou, W., Jiang, Y., He, S., & Chen, D. (2010). Olfaction modulates visual perception in binocular rivalry. Current Biology, 20(15), 1356–1358.
Acknowledgements
Supported by the National Science Foundation grants 1116988 and 1526448.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 333 kb)
Rights and permissions
About this article
Cite this article
Paris, R., Bodenheimer, B. & Blake, R. Does direction of walking impact binocular rivalry between competing patterns of optic flow?. Atten Percept Psychophys 79, 1182–1194 (2017). https://doi.org/10.3758/s13414-017-1299-4
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13414-017-1299-4