Abstract
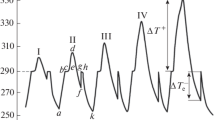
Overcooling of gallium–tin alloys under normal conditions has been studied by thermal analysis. The following samples have been analyzed: Ga (I); two hypoeutectic alloys: 95% Ga + 5% Sn (II), 90% Ga + 10% Sn (III); eutectic alloy: 96.3% Ga + 13.7% Sn (IV); and five hypereutectic alloys with Sn content of 20% (V), 35% (VI), 50% (VII), 80% (VIII), and pure tin (IX). A nonequilibrium state diagram of this system is constructed. Herewith, the eutectic composition does not vary and the eutectic temperature decreases to 5.5°C, that is, 26°C below that of three-phase eutectic equilibrium. The eutectic temperature does not actually vary upon a variation of cooling rates of eutectic alloy from 0.06 to 60°C/min. It has been detected that a slight decrease in overcooling is expected in the hypoeutectic region, whereas in the hypereutectic region overcooling increases while the alloy composition approaches eutectic. Activities and activity coefficients of components on the lines of equilibrium and nonequilibrium liquidus have been calculated. It is demonstrated that the activities on the lines of both equilibrium and nonequilibrium liquidus decrease in a predictable manner, and the activity coefficients increase while the composition approaches eutectic. Concentration paths of equilibrium and nonequilibrium crystallization are shown in the state diagrams.


Similar content being viewed by others
REFERENCES
Liang Zhao, Yuming Xing Ze, and Wang Xin Liu, The passive thermal management system for electronic device using low-melting-point alloy as phase change material, Appl. Therm. Eng., 2017, vol. 125, pp. 317–327. https://doi.org/10.1016/j.applthermaleng.2017.07.004
Krayukhin, V.I., RF Patent C09K3/10, 2009. Krayukhin, V.I., RF Patent 2345865 C2, 2009.
Roy, Ch.K., Bhavnani, S., Hamilton, M.C., Wayne, J.R., Knight, R.W., and Harris, D.K., Thermal performance of low melting temperature alloys at the interface between dissimilar materials, Appl. Therm. Eng., 2016, vol. 99, pp. 72–79. https://doi.org/10.1016/j.applthermaleng.2016.01.036
Chentsov, V.P., Shevchenko, V.G., Mozgovoi, A.G., and Pokrasin, M.A., Density and surface tension of heavy liquid-metal coolants: gallium and indium, Inorg. Mater.: Appl. Res., 2011, vol. 2, no. 5, pp. 468–473.
Ivanova, A.G. and Gerasimov, S.F., Dependence of the phase transition temperature of the eutectic alloy Ga‒Zn on its morphology, Izmer. Tekh., 2009, no. 1, pp. 34–37.
Diagrammy sostoyaniya dvoinykh metallicheskikh system. Spravochnik (State Diagrams of Binary Metal Systems. Handbook), Lyakishev, N.P., Ed., Moscow: Mashinostroenie, 1997, vol. 2, pp. 657–658.
Puschin, N.A., Stepanoviĉ, S., and Stajiĉ, V., Über die Legierungen des Galliums mit Zink, Cadmium, Quecksilber, Zinn, Blei, Wismut und Aluminium, Z. Anorg. Allg. Chem., 1932, vol. 209, no. 3, p. 329.
Predel, B., Zustandsdiagramm und eigenschaften von Gallium-Zinn-Legierungen, J. Less-Common Met., 1964, vol. 7, no. 5, pp. 347–355.
Trebuhov, A.A., Sarmurzina, R.K., and Sokolskii, D.V., Study of the physicochemical properties of the gallium-tin system, Zh. Fiz. Khim., 1985, no. 8, pp. 2065–2067.
Brekharya, G.P., Effect of cooling rate onto supercooling of metals and alloys and structure formation, Extended Abstract of Cand. Sci. (Eng.) Dissertation, Dnepropetrovsk: Dnepropetrovsk State Univ., 1976.
Aleksandrov, V.D., Kinetika zarodysheobrazovaniya i massovoi kristallizatsii pereokhlazhdennykh rasplavov i amorfnykh sred (Kinetics of Nucleation and Mass Crystallization of Supercooled Liquids and Amorphous Media), Donetsk: Donbass, 2011.
Aleksandrov, V.D., UA Patent 83721, 2008.
Perepezko, J.H., Nucleation in undercooled liquids, Mater. Sci. Eng., 1984, vol. 65, no. 1, pp. 125–135.
Šesták, J., Thermal Analysis: Thermophysical Properties of Solids: their Measurements and Theoretical Thermal Analysis, Amsterdam, New York: Elsevier Science, 1984.
GOST (State Standard) no. R532933–2009: Identification of Substances and Materials (Heating and Cooling Curves for TA, DTA, DSC, TGA), 2009.
Aleksandrov, V.D. and Frolova, S.A., Effect of the overheating of the gallium melt on its supercooling during solidification, Russ. Metall. (Engl. Transl.), 2014, vol. 2014, no. 1, pp. 14–19. https://doi.org/10.1134/S0036029514010042
Aleksandrov, V.D. and Frolova, S.A., The effect of thermal processing of the liquid phase on the crystallization of alloys in the Sn–Bi system, Rasplavy, 2003, no. 3, pp. 14–21.
Aleksandrov, V.D. and Barannikov, V.D., Study of the influence of thermal history of tin and lead drops on their crystallization by cyclic thermal analysis, Khim. Fiz., 1998, vol. 17, no. 10, pp. 140–147.
Aleksandrov, V.D., Frolova, S.A., and Amerkhanova, Sh.K., Solidification of the eutectic Ga–Sn alloy, Russ. Metall. (Engl. Transl.), 2016, vol. 2016, no. 5, pp. 437–442. https://doi.org/10.1134/S0036029516050025
Er-Guang Jia, Ai-Qing Wu, Li-Jun Guo, Liu, C.S., Wen-Jun Shan, and Zhen-Gang Zhu, Experimental evidence of the transformation from microheterogeneous to microhomogeneous states in Ga-Sn melts, Phys. Lett. A, 2007, vol. 364, no. 6, pp. 505–509. https://doi.org/10.1016/j.physleta.2006.12.048
Zhao Xiaolin, Bian Xiufang, Wang Changchun, and Li Yunfang, The evolution of coordination structure in liquid GaSn alloy, Chin. J. Phys., 2018, vol. 56, no. 6, pp. 2684–2688. https://doi.org/10.1016/j.cjph.2018.10.025
Stromberg, A.G. and Semchenko, D.P., Fizicheskaya khimiya (Physical Chemistry), Moscow: Vysshaya Shkola, 1973.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that the presented data do not contain any conflict of interest.
Additional information
Translated by I. Moshkin
About this article
Cite this article
Aleksandrov, V.D., Zozulia, A.P. & Frolova, S.A. Construction of Gallium–Tin Nonequilibrium State Diagram and Its Analysis. Russ. J. Non-ferrous Metals 61, 172–178 (2020). https://doi.org/10.3103/S1067821220020029
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1067821220020029