Abstract
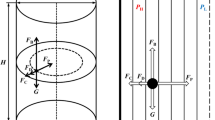
The joint consideration of subprocesses of particle capture and detachment, and buoying of aggregates in the periodic nonfrothing flotation conditions shows that the mineral load formed on a separate part for its buoying time (τm). This load is a part of the equilibrium mineral load, which can be attained under the infinite mineralization time. It is proposed to characterize the composition and attainment rate of the mineral load by two dimensionless parameters, which depend on intensities of subprocesses. The sort parameter of particles (B) has been uniquely determined by the ratio of the detachment intensity to the capture intensity, while the dimensionless time (D) is determined by the ratio of the particle capture and detachment rate to the buoying velocity of the air bubble. The mineralization kinetic equation by many bubbles is derived in the exponential form similarly to the first-order Beloglazov equation. Intensities of capture and detachment subprocesses in the mineralization rate constant (K m) determine the magnitude of recovery by a separate bubble (εbm) for time τm, while the air consumption determines the summary recovery ε.
Similar content being viewed by others
References
Zheng, X., Johnson, N.W., and Franzidis, J.P., Modelling of entrainment in industrial flotation cells: water recovery and degree of entrainment, Miner. Eng., 2006, vol. 19, no. 11, pp. 1191–1203.
Yianatos, J., Contreras, F., Diaz, F., and Villanueva, A., Direct measurement of entrainment in large flotation cells, Powder Technol., 2009, vol. 189, no. 1, pp. 42–47.
Dobby, G.S. and Finch, J.A., Particle size dependence in flotation derived from a fundamental model of the capture process, Int. J. Miner. Process., 1987, vol. 21, pp. 241–253.
Dai, Z., Fornasiero, D., and Ralston, J., Particle-bubble collision models: A review, Adv. Colloid Interface Sci., 2000, vol. 85, pp. 231–256.
Yianatos, J., Bucarey, R., Larenas, J., Henriquez, F., and Torres, L., Collection zone kinetic model for industrial flotation columns, Miner. Eng., 2005, vol. 18, pp. 1373–1377.
Duan, J., Fornasiero, D., and Ralston, J., Calculation of the flotation rate constant of chalcopyrite particles in an ore, Int. J. Miner. Process., 2003, vol. 72, pp. 227–237.
Samygin, V.D., Filippov, L.O., and Shekhirev, D.V., Osnovy obogashcheniya rud Foundations of the Ore Concentration), Moscow: Al’teks, 2003.
Bogdanov, O.S., Maximov, I.I., Podnek, A.K., and Yanis, N.A., Teoriya i tekhnologia flotatsii rud (Theory and Technology of Ore Flotation), Moscow: Nedra, 1990.
Tikhonov, O.N., Teoriya razdeleniya mineralov. Uchebnik (Separation Theory of Minerals. Textbook), St. Petersburg: St. Petersburg Gornyi Univ., 2008.
Mika, T. and Fuerstenau, D., Microscopy model of flotation process, in: VIII Mezhdunarodnyi congress po obogashcheniyu poleznykh iskopaemykh (VIII Int. Mineral Processing Congr.), vol. 2, Leningrad, 1969, pp. 246–269.
Rubinshtein, J.B., Effect of particle and bubble size on flotation kinetics, in Frothing in Flotation, London, New York: Gordon and Breath, 1998, vol. 2, pp. 51–80.
Saleh, A.M., A study on the performance of second order models and two phase models in iron ore flotation, Physicochem. Probl. Miner. Process., 2010, vol. 44, pp. 215–230.
Shekhirev D.V., Filipov L.O., Samygin V.D. Mathematical modelling of the process of separation of the raw materials in the column flotation, in: Proc. XVIII Intern. Mineral Processing Congr., Sydney: Aus. IMM, pp. 1357–1362.
Abramov, A.A., Din’ Ngok Dang, and Ivanov, B.A., The probabilistic concept of the flotation process, Izv. Vyssh. Uchebn. Zaved., Gornyi Zh., 1978, no. 3, pp. 153–158.
Koh, P.T.L. and Schwarz, M.P., CFD modelling of bubble-particle collision rates and efficiencies in mineral flotation cells, Miner. Eng., 2003, vol. 16, pp. 1055–1059.
Koh, P.T.L. and Schwarz, M.P., CFD model of a selfaerating flotation cell, Int. J. Miner. Process., 2007, vol. 85, no. 3, pp. 16–24.
Koh, P.T.L. and Schwarz, M.P., Modelling attachment rates of multi-sized bubbles with particles in a flotation cell, Miner. Eng., 2008, vol. 21, pp. 989–993.
Koh, P.T.L. and Smith, L.K., The effect of stirring speed and induction time on flotation, Miner. Eng., 2011, vol. 24, no. 5, pp. 442–448.
Huang, Z., Legendre, D., and Guiraud, P., Effect of interface contamination on particle-bubble collision, Chem. Eng. Sci., 2012, vol. 68, no. 1, pp. 1–18.
Bocharov, V.A., Ignatkina, V.A., and Alekseichuk, D.A., Influence of mineral compositions and their modification on the selection flowchart and collectors of selective flotation of ores of nonferrous metals, Russ. J. Non-Ferrous Met., 2012, vol. 53, pp. 279–288.
Samygin, V.D. and Grigoryev, P.V., Modeling of the influence of the hydrodynamic factors on the flotation process. Pt. 1. Influence of the bubble diameter and turbulent energy dissipation, Fiz.-Tekh. Probl. Razrab. Polezn. Iskop., 2015, no. 1, pp. 1–8.
Arbiter, N., Flotation rate and flotation efficiency, Miner. Eng., 1951, vol. 190, no. 3, pp. 791–796.
Yianatos, J., Bucarey, R., Larenas, J., Henriquez, F., and Torres, L., Collection zone kinetic model for industrial flotation columns, Miner. Eng., 2005, vol. 18, pp. 1373–1377.
Barskii, L.A. and Kozin, V.Z., Sistemnyi analiz v obogashchenii poleznykh iskopaemykh (System Analysis in Concentration of Minerals), Moscow: Nedra, 1978.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © V.D. Samygin, 2016, published in Izvestiya Vysshikh Uchebnykh Zavedenii, Tsvetnaya Metallurgiya, 2016, No. 3, pp. 4–11.
About this article
Cite this article
Samygin, V.D. Mineralization kinetics of air bubbles allowing for the particle detachment and time of buoying of aggregates. Russ. J. Non-ferrous Metals 57, 389–394 (2016). https://doi.org/10.3103/S1067821216050199
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1067821216050199