Abstract
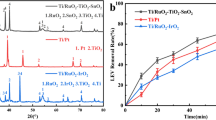
The sol-gel approach was utilized to make a copper-doped Ti/SnO2-Sb anode for electrochemical oxidation of norfloxacin (NOR). Sycanning electron microscopy (SEM), energy dispersive spectrometry (EDS), X-ray diffraction (XRD), linear scan voltammograms (LSV), and cyclic voltammetry (CV) were used to examine the anode’s surface morphology and electrochemical characteristics. The results show that the doped metal makes the anode coating denser and smoother, which is more conducive to resistance to electrochemical corrosion. The electrochemical degradation of NOR utilizing the constructed anode was examined in relation to reaction circumstances such as current density, pH value, and initial NOR concentration. Within the selected range of reaction conditions, the removal rate of NOR using Ti/SnO2–Sb–Cu electrode was significantly higher than that of blank electrode. The following were the shifting trends: NOR removal efficiencies grew with applied current density and initial NOR concentration, and reduced with increasing pH value. Water samples with different reaction times were taken and tested by UV-Vis spectrum. As can be seen from the results, with the progress of the reaction, the intensity of the characteristic peak decreased, indicating that NOR is degraded gradually. The electrochemical degradation pathways of NOR were studied using liquid chromatography coupled with mass spectrometry (LC-MS). Six intermediates were detected and a possible reaction pathway for the degradation of NOR by electrochemical oxidation was proposed.











Similar content being viewed by others
REFERENCES
Feifei, L., Lyujun, C., Weidong, C., and Yingyu, B., Antibiotics in coastal water and sediments of the East China Sea: Distribution, ecological risk assessment and indicators screening, Mar. Pollut. Bull., 2020, vol. 151, p. 110810.
Yinzhi, L., Xiaojun, L., Jianliang, Z., Shanquan, W., and Bixian, M., Occurrence and distribution of antibiotics in sediments from black-odor ditches in urban areas from China, Sci. Total Environ., 2021, vol. 787, p. 147554.
Chen, L., Venkateswarlu, N., Gregory, V.K., and Wenjun, J., Spectroscopic study of degradation products of ciprofloxacin, norfloxacin and lomefloxacin formed in ozonated wastewater, Water Res., 2012, vol. 46, pp. 5235–5246.
Mingjie, H., Tao, Z., Xiaohui, W., and Juan, M., Distinguishing homogeneous-heterogeneous degradation of norfloxacin in a photochemical Fenton-like system (Fe3O4/UV/oxalate) and the interfacial reaction mechanism, Water Res., 2017, vol. 119, pp. 47–56.
Larsson, D.G.J., de Pedro, C., and Paxeus, N., Effluent from drug manufactures contains extremely high levels of pharmaceuticals, J. Hazard. Mater., 2007, vol. 148, pp. 751–755.
Fick, J., Söderström, H., Lindberg, R.H., Phan, C., Tysklind, M., and Larsson, D.G.J., Contamination of surface, ground, and drinking water from pharmaceutical production, Environ. Toxicol. Chem., 2009, vol. 28, pp. 2522–2527.
Salazar, C., Contreras, N., Mansilla, H.D., Yáñez, J., and Salazar, R., Electrochemical degradation of the antihypertensive losartan in aqueous medium by electro-oxidation with boron-doped diamond electrode, J. Hazard. Mater., 2016, vol. 319, pp. 84–92.
Radjenovic, J. and Sedlak, D.L., Challenges and opportunities for electrochemical processes as next-generation technologies for the treatment of contaminated water, Environ. Sci. Technol., 2015, vol. 49, pp. 11292–11302.
Särkkä, H., Bhatnagar, A., and Sillanpää, M., Recent developments of electro-oxidation in water treatment: A review, J. Electroanal. Chem., 2015, vol. 754, pp. 46–56.
Coledam, D.A.C., Aquino, J.M., Silva, B.F., Silva, A.J., and Rocha-Filho, R.C., Electrochemical mineralization of norfloxacin using distinct boron-doped diamond anodes in a filter-press reactor, with investigations of toxicity and oxidation by-products, Electrochim. Acta, 2016, vol. 213, pp. 856–864.
Sun, Y.Y., Zhang, C.Y., Rong, H.Y., Wu, L., Lian, B.Y., Wang, Y., Chen, Y., Tu, Y., and Waite, T.D., Electrochemical Ni-EDTA degradation and Ni removal from electroless plating wastewaters using an innovative Ni-doped PbO2 anode: Optimization and mechanism, J. Hazard. Mater., 2022, vol. 424, p. 127655.
Fan, J., Zhao, G., Zhao, H., Chai, S., and Cao, T., Fabrication and application of mesoporous Sb-doped SnO2 electrode with high specific surface in electrochemical degradation of ketoprofen, Electrochim. Acta, 2013, vol. 94, pp. 21–29.
Wang, Y., Shen, Ch., Zhang, M., Zhang, B.-T., and Yu, Y.-G., The electrochemical degradation of ciprofloxacin using a SnO2-Sb/Ti anode: Influencing factors, reaction pathways and energy demand, Chem. Eng. J., 2016, vol. 296, pp. 79–89.
Li, L., Huang, Z., Fan, X., Zhang, Z., Dou, R., Wen, S., Chen, Y., Chen, Y., and Hu, Y., Preparation and characterization of a Pd modified Ti/SnO2-Sb anode and its electrochemical degradation of Ni-EDTA, Electrochim. Acta, 2017, vol. 231, pp. 354–362.
Chen, Y., Li, F., Dong, X., Guo, D., Huang, Y., and Li, S., Construction of rGO@Ti/SnO2-Sb composite electrode for electrochemical degradation of fluoroquinolone antibiotic, J. Alloy. Compd., 2021, vol. 869, p. 159258.
Meng, J., Li, D., Zhang, L., Gao, W., Huang, K., Geng, C., Guan, Y., Ming, H., Jiang, W., and Liang, J., Degradation of norfloxacin by electrochemical oxidation using Ti/SnO2-Sb electrode doped with Ni or Mo, Electrocatalysis, 2021, vol. 12, pp. 436–446.
Liang, J., Geng, C., Li, D., Cui, L., and Wang, X., Preparation and degradation phenol characterization of Ti/SnO2-Sb-Mo electrode doped with different contents of molybdenum, J. Mater. Sci. Technol., 2015, vol. 31, pp. 473–478.
Wang, Y.-H., Li, G., Chen, Q.-Y., Geng, X., and Yan, W., Effect of drying periods on antimony-doped tin dioxide-coated titanium electrode, J. Solid. State Electrochem., 2013, vol. 17, pp. 1985–1989.
Li, S., Wang, W., Zeng, X., and Ma, X., Electro-catalytic degradation mechanism of nitenpyram in synthetic wastewater using Ti-based SnO2-Sb with rare earth-doped anode, Desalin. Water Treat., 2015, vol. 54, pp. 1925–1938.
Cheng, W., Yang, M., Xie, Y., Fang, Z., Nan, J., and Tsang, P.E., Electrochemical degradation of the antibiotic metronidazole in aqueous solution by the Ti/SnO2-Sb-Ce anode, Environ. Technol., 2013, vol. 34, pp. 2977–2987.
Li, D., Tang, J., Zhou, X., Li, J., Sun, X., Shen, J., Wang, L., and Han, W., Electrochemical degradation of pyridine by Ti/SnO2-Sb tubular porous electrode, Chemosphere, 2016, vol. 149, pp. 49–56.
Song, S., Fan, J., He, Z., Zhan, L., Liu, Zh., Chen, J., and Xu, X., Electrochemical degradation of azo dye C.I. Reactive Red 195 by anodic oxidation on Ti/SnO2-Sb/PbO2 electrodes, Acta Electrochim., 2010, vol. 55, pp. 3606–3613.
Zhi, D., Qin, J., Zhou, H., Wang, J., and Yang, Sh., Removal of tetracycline by electrochemical oxidation using a Ti/SnO2–Sb anode: Characterization, kinetics, and degradation pathway, J. Appl. Electrochem., 2017, vol. 47, pp. 1313–1322.
Niu, J., Maharana, D., Xu, J., Chai, Z., and Bao, Yu., A high activity of Ti/SnO2-Sb electrode in the electrochemical degradation of 2,4-dichlorophenol in aqueous solution, J. Environ. Sci., 2013, vol. 25, pp. 1424–1430.
Özcan, A., Özcan, A.A., and Demirci, Y., Evaluation of mineralization kinetics and pathway of norfloxacin removal from water by electro-Fenton treatment, Chem. Eng. J., 2016, vol. 304, pp. 518–526.
Funding
This work was supported by the LiaoNing Revitalization Talents Program (XLYC1908034, XLYC2007170, Key R&D project of Liaoning Province of China (no. 2020JH2/10300079) and Scientific research fund project of Liaoning Provincial Department of Education (LQGD2020014).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflicts of interest.
Supplementary Information
About this article
Cite this article
Jing Meng, Geng, C., Guan, Y. et al. Ultra-Fast Electrochemical Oxidation of Norfloxacin on Copper-Doped Ti/SnO2–Sb Anodes: Influencing Factors and Degradation Pathways. J. Water Chem. Technol. 45, 164–175 (2023). https://doi.org/10.3103/S1063455X23020066
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1063455X23020066