Abstract
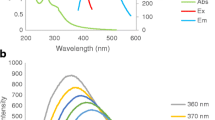
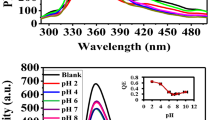
In this work, we have developed a near-infrared emissive polyethyleneimine capped Ag2(PEI-Ag2S QD) quantum dots based chemosensor for sensing trace-level Hg2+ in water by taking advantage of the metal-induced aggregation strategy. The fluorescence determination of Hg2+ by PEI-Ag2S QD at different pH (pH 4, 7, and 10) were performed, respectively. Under the optimum conditions, the selectivity of this system for Hg2+ over other metal ions in aqueous solutions was remarkably high, and its detection limits was 0.5 nM at pH 4. In addition, the present approach provides the advantages of rapidity, simplicity, low background and low cost. The assay also offers great potential for specific detection of Hg2+ in real water samples. This developed PEI-Ag2S QD-based method presages more opportunities for application in environmental systems.




Similar content being viewed by others
REFERENCES
Renzoni, A., Zino, F., and Franchi, E., Mercury levels along the food chain and risk for exposed populations, Environ. Res., 1998, vol. 77, no. 2, pp. 68–72.
Harris, H.H., Pickering, J., and George, G.N., The chemical form of mercury in fish, Science, 2003, vol. 301, pp. 1203–1208.
Ghaedi, M., Fathi, M.R., Shokrollahi, A., and Shajarat, F., Highly selective and sensitive preconcentration of mercury ion and determination by cold vapor atomic absorption spectroscopy, Anal. Lett., 2006, vol. 39, pp. 1171–1185.
Zhao, Y., Zheng, J., Fang, L., Lin, Q., Wu, Y., Xue, Z., and Fu, F., Speciation analysis of mercury in natural water and fish samples by using capillary electrophoresis–inductively coupled plasma mass spectrometry, Talanta, 2012, vol. 89, pp. 280–285.
Li, Y., Chen, C., Li, B., Sun, J., Wang, J., Gao, Y., et al., Elimination efficiency of different reagents for the memory effect of mercury using ICP-MS, J. Anal. At. Spectrom., 2006, vol. 21, pp. 94–96.
Bhanjana, G., Dilbaghi, N., Kumar, R., and Kumar, S., Zinc oxide quantum dots as efficient electron mediator for ultrasensitive and selective electrochemical sensing of mercury, Electrochim. Acta, 2015, vol. 178, pp. 361–367.
Leopold, K., Foulkes, M., and Worsfold, P.J., Gold-coated silica as a preconcentration phase for the determination of total dissolved mercury in natural waters using atomic fluorescence spectrometry, Anal. Chem., 2009, vol. 81, pp. 3421–3428.
Zi, H.G., Gan, W.E., Han, S.P., Jiang, X.J., and Wan, L.Z., Line sorption preconcentration-cold vapor atomic fluorescence spectrometry, Chin. J. Anal. Chem., 2009, vol. 7, pp. 1029–1032.
Eftekhari, E., Wang, W., Li, X., Nikhil, A., Wu, Z., Klein, R., et al., Picomolar reversible Hg(II) solid-state sensor based on carbon dots in double heterostructure colloidal photonic crystals, Sens. Actuators, B, 2017, vol. 240, pp. 204–210.
Chansuvarn, W., Tuntulani, T., and Imyim, A., Colorimetric detection of mercury(II) based on gold nanoparticles, fluorescent gold nanoclusters and other gold-based nanomaterials, TrAC,Trends Anal. Chem., 2015, vol. 65, pp. 83–96.
Fu, A.H., Gu, W.W., Larabell, C., and Alivisatos, A.P., Semiconductor nanocrystals for biological imaging, Curr. Opin. Neurobiol., 2005, vol. 15, pp. 568–575.
Dai, Z., Zhang, J.M., Dong, Q.X., Guo, N., Xu, S.C., Sun, B., and Bu, Y.H., Novel sorbents for mercury emissions control from coal-fired power plants, J. Chin. Inst. Chem. Eng., 2007, vol. 15, pp. 791–794.
Hu, T., Yan, X., Na, W., and Su, X., Aptamer-based aggregation assay for mercury(II) using gold nanoparticles and fluorescent CdTe quantum dots, Microchim. Acta, 2016, vol. 183, no. 7, pp. 2131–2137.
Xia, Y.S. and Zhu, C.Q., Use of surface-modified CdTe quantum dots as fluorescent probes in sensing mercury (II), Talanta, 2008, vol. 75, no. 1, pp. 215–221.
Ke, J., Li, X.Y., Shi, Y., Zhao, Q.D., and Jiang, X.C., A facile and highly sensitive probe for Hg(II) based on metal-induced aggregation of ZnSe/ZnS quantum dots, Nanoscale, 2012, vol. 4, pp. 4996–5001.
Li, Z.Z., Zhang, Q.Y., Huang, H.Y., Ren, C.J., Pan, Y.J., Wang, Q., and Zhao, Q., RGDS-conjugated C-dSeTe/CdS quantum dots as near-infrared fluorescent probe: preparation, characterization and bioapplication, J. Nanopart. Res., 2016, vol. 18, no. 12, pp. 373–388.
Buffet, P.E., Zalouk-Vergnoux, A., Poirier, L., Lopes, C., Risso-de-Faverney, C., Guibbolini, M., et al., Cadmium sulfide quantum dots induce oxidative stress and behavioral impairments in the marine clam Scrobicularia plana,Environ. Toxicol. Chem., 2015, vol. 34, no. 7, pp. 1659–1664.
Liu, L., Zhang, J., Su, X., and Mason, R.P., In vitro and In vivo assessment of CdTe and CdHgTe toxicity and clearance, J. Biomed. Nanotechnol., 2008, vol. 4, no. 4, pp. 524–528.
Kim, J.H., Lee, B.R., Choi, E.S., Kim, E., and Kim, H.R., Carcinogenic activity of PbS quantum dots screened using exosomal biomarkers secreted from HEK293 cells, Int. J. Nanomed., 2015, vol. 10, pp. 5513–5528.
O’Hara, T., Seddon, B., O’Connor, A., McClean, S., Singh, B., Iwuoha, E., et al., Quantum dot nanotoxicity investigations using human lung cells and TOXOR electrochemical enzyme assay methodology, ACS Sens., 2017, vol. 2, no. 1, pp. 165–171.
Wang, C.X., Wang, Y., Xu, L., Zhang, D., Liu, M.X., Li, X.W., et al., Facile aqueous-phase synthesis of biocompatible and fluorescent Ag2S nanoclusters for bioimaging: tunable photoluminescence from red to near infrared, Small, 2012, vol. 8, no. 20, pp. 3137–3142.
Duman, F.D., Hocaoglu, I., Ozturk, D.G., Gozuacik, D., Kiraza, A., and Acar, H.Y., Highly luminescent and cytocompatible cationic Ag2S NIR-emitting quantum dots for optical imaging and gene transfection, Nanoscale, 2015, vol. 7, no. 26, pp. 11352–11362.ììì
Luo, J.D., Xie, Z.L., Lam, J.W.Y., Cheng, L., Chen, H.Y., Qiu, C.F., et al., Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole, Chem. Commun., 2001, vol. 18, pp. 1740–1741.
Ding, D., Li, K., Liu, B., and Tang, B.Z., Bioprobes based on AIE fluorogens, Acc. Chem. Res., 2013, vol. 46, no. 11, pp. 2441–2453.
Ding, Y.B., Shi, L.L., and Wei, H.A., A “turn on” fluorescent probe for heparin and its oversulfated chondroitin sulfate contaminant, Chem. Sci., 2015, vol. 6, pp. 6361–6366.
Yan, D., He, Y., Ge, Y.L., and Song, G.W., Fluorescence “turn on-off” detection of heparin and heparinase I based on the near-infrared emission polyethyleneimine capped Ag2S quantum dots, Sens. Actuators, B, 2017, vol. 240, pp. 863–869.
Wang, L., Li, B.Q., and Xu, F., High-yield synthesis of strong photoluminescent N-doped carbon nanodots derived from hydrosoluble chitosan for mercury ion sensing via smartphone APP, Biosens. Bioelectron., 2016, vol. 79, pp. 1–8.
Wang, P. and Zhao, L.Y., Dual-emitting fluorescent chemosensor based on resonance energy transfer from poly (arylene ether nitrile) to gold nanoclusters for mercury detection, Sens. Actuators, B, 2016, vol. 230, pp. 337–344.
Fang, Q. and Liu, Q., An aqueous fluorescent probe for Hg2+ detection with high selectivity and sensitivity, Luminescence, 2015, vol. 30, pp. 1280–1284.
Yang, R. and Ding, X.J., A novel fluorescent sensor for mercury (II) ion using self-assembly of poly(diallyl dimethyl ammonium)chloride functionalized CdTe quantum dots, Anal. Methods, 2015, vol. 7, no. 2, pp. 436–442.
Li, N. and Dai, J.K., β-Carboline-functionalized dithioacetal as Hg2+-selective fluorescence probe in water, Spectrochim. Acta, 2015, vol. 136, pp. 900–905.
Ma, Y.H., Zhang, Z., Xu, Y.L., Ma, M., Chen, B., Wei, L., and Xiao, L.H., A bright carbon-dot-based fluorescent probe for selective and sensitive detection of mercury ions, Talanta, 2016, vol. 161, pp. 476–481.
Zhu, R. and Zhou, Y., Detection of Hg2+ based on the selective inhibition of peroxidase mimetic activity of BSA-Au clusters, Talanta, 2013, vol. 117, pp. 127–132.
Isaad, J. and Achari, E.A., Azathia crown ether possessing a dansyl fluorophore moiety functionalized silica nanoparticles as hybrid material for mercury detection in aqueous medium, Tetrahedron, 2013, vol. 69, no. 24, pp. 4866–4874.
Yantasee, W., Lin, Y.H., Zemanian, T.S., and Fryxell, G.E., Voltammetric detection of lead(II) and mercury(II) using a carbon paste electrode modified with thiol self-assembled monolayer on mesoporous silica (SAMMS), Analyst, 2003, vol. 128, no. 5, pp. 467–472.
Funding
This work was financially supported by Supported by National Natural Science Foundation of China (21707030) and Wuhan Youth Science and technology plan (2016070204010133).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
You, J.Q., Yan, D., He, Y. et al. Polyethyleneimine-Protected Ag2S Quantum Dots for Near-Infrared Fluorescence-Enhanced Detection of Trace-Level Hg2+ in Water. J. Water Chem. Technol. 42, 36–44 (2020). https://doi.org/10.3103/S1063455X20010105
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1063455X20010105