Abstract
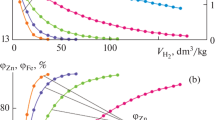
The paper presents the thermodynamic modeling results of zinc and iron reduction from B2O3–CaO–Fe2O3–ZnО melts by CO–CO2 and H2–H2O mixtures containing 0–60% CO2 (H2O) at 1273–1673 K using a technique describing the reduction of metals from an oxide melt by gas in bubbling processes, under conditions that provide an approximation to real systems. Its originality is equilibrium determination for each individual portion of gas supplied into the working fluid. The reducible metals oxides content in each calculation cycle is taken from the previous data. During the calculations, changes in the content of zinc (СZnO) and iron (\({{C}_{{{\text{F}}{{{\text{e}}}_{2}}{{{\text{O}}}_{3}}}}},\) \({{C}_{{{\text{F}}{{{\text{e}}}_{3}}{{{\text{O}}}_{4}}}}}\) and СFeO) oxides in the melt and the degree of their reduction were estimated. When using CO or H2 as a reducing agent, this process proceeds in three stages. In the first stage, Fe2O3 is reduced to Fe3O4 and FeO. \({{C}_{{{\text{F}}{{{\text{e}}}_{2}}{{{\text{O}}}_{3}}}}}\) values decrease to almost zero, while \({{C}_{{{\text{F}}{{{\text{e}}}_{3}}{{{\text{O}}}_{4}}}}}\) and CFeO increase simultaneously. By the end of the stage, \({{C}_{{{\text{F}}{{{\text{e}}}_{3}}{{{\text{O}}}_{4}}}}}\) reaches its maximum value. At the second stage, the Fe3O4 → FeO transition occurs, when СFeO values reach its maximum. At these stages, there is a slight increase in the CZnO. At the third stage, the values CFeO and CZnO decrease, and iron and zinc are reduced. An increase in temperature dramatically reduces the gas consumption for zinc reduction by 2–3 times, and the replacement of CO with H2 reduces it by less than 20%. In the presence of oxidizing agents (CO or H2O), only zinc is reduced. The process ends when the final content of zinc oxide in the melt corresponds to the equilibrium with the initial gas composition. The higher the temperature, the less CZnO is. The obtained data are useful for the development of technologies for the selective recovery of metals.




Similar content being viewed by others
REFERENCES
Wang, C., Li, K., Yang, H., and Li, C., Probing study on separating Pb, Zn, and Fe from lead slag by coal-based direct reduction, ISIJ Int., 2017, vol. 57, no. 6, pp. 996–1003. https://doi.org/10.2355/isijinternational.ISIJINT-2016-683
Leont’ev, L.I. and Dyubanov, V.G., Technogenic waste of ferrous and non-ferrous metallurgy and environmental problems, Ekol. Promyshl. Ross., 2011, no. 4, pp. 32–35.
Yakornov, S.A., Pan’shin, A.M., Kozlov, P.A., and Ivakin, D.A., Development of technology and instrumental scheme of pyrometallurgical processing of ferrous metallurgy dusts, Tsvetn. Met., 2017, no. 9, pp. 39–44. https://doi.org/10.17580/tsm.2017.09.06
Gorlova, O.E., Tarasova, A.E., and Efremova, O.G., Finding ways of complex processing of blast furnace sludge, Vestn. Magnitogorskogo Gos. Tekh. Univ. im G.I. Nosova, 2005, no. 4, pp. 4–6.
Guezennec, A.-G., Huber, J.-C., Patisson, F., Sessieq, P., Birat, J.-P., and Ablitzer, D., Dust formation in electric arc furnace: Birth of the particles, Powder Technol., 2005, vol. 157, nos. 1–3, pp. 2–11. https://doi.org/10.1016/j.powtec.2005.05.006
Okunev, A.I., Kost’yanovskii, I.A., and Donchenko, P.A., F’yumingovanie shlakov (Slag Fuming), Moscow: Metallurgiya, 1966.
Tarasov, A.V., Besser, A.D., and Mal’tsev, V.I., Metallurgicheskaya pererabotka vtorichnogo tsinkovogo syr’ya (Metallurgical Processing of Secondary Zinc Raw Materials), Moscow: GINTSVETMET, 2004.
Kozlov, P.A., Development of recycling of metallurgical technogenic waste, Tsvet. Metall., 2014, no. 2, pp. 45–52.
Reddy, R.G., Prabhu, V.L., and Mantha, D., Zinc fuming from lead blast furnace slag, High Temp. Mater. Process., 2003, vol. 21, no. 6, pp. 377–386. https://doi.org/10.1515/HTMP.2002.21.6.377
Verscheure K., van Camp M., Blanpain B., Wollants P., Hayes P.C., and Jak E., Zinc fuming processes for treatment of zinc containing residues, Proc. of Lead and Zinc, Osaka, 2005, MMIJ, 2005, pp. 943–960.
Morcali, M.H., Yucel, O., Aydin, A., and Derin, B., Carbothermic reduction of electric arc furnace dust and calcination of Waelz oxide by semi-pilot scale rotary furnace, J. Min. Metall., Sect. B: Metall., 2012, vol. 48, no. 2, pp. 173–184. https://doi.org/10.2298/JMMB111219031M
Zhang, H.N., Li, J.L., Xu, A.J., Yang, Q.X., He, D.F., and Tian, N.Y., Carbothermic reduction of zinc and iron oxides in electric arc furnace dust, J. Iron Steel Res. Int., 2014, vol. 21, no. 4, pp. 427–432. https://doi.org/10.1016/S1006-706X(14)60066-2
Tyushnyakov, S.N., Selivanov, E.N., and Chumarev, V.M., Estimation of rate of zinc distillation from slag in direct-current ARC furnace, Tsvetn. Met., 2013, no. 12, pp. 13–17.
Kozyrev, V.V., Distillation of zinc from slag during fuming by natural gas, Tsvetn. Met., 2009, no. 2, pp. 61–64.
Kozyrev, V.V., Besser, A.D., and Paretskii, V.M., On zinc extraction from lead smelting slags, Elektrometallurgiya, 2013, no. 6, pp. 31–35.
Romenets, V.A., Romelt process, Iron Steelmaker, 1995, vol. 22, no. 1, pp. 37–41.
Dorofeev, G.A., Yantovskii, P.R., Smirnov, K.G., and Stepanov, Ya.M., The process “orien” for smelting of high-quality steels from ore and energy raw materials based on the principle of the energy self-supplying, Chern. Met., 2017, no. 5, pp. 17–23.
Schlesinger, M.E., King, M.J., Sole, K.C., and Davenport, W.G., Extractive Metallurgy of Copper, Elsevier, 2011, 5th ed.
Vignes, A., Extractive Metallurgy 3: Processing Operations and Routes ISTE Ltd., John Wiley & Sons, 2011.
Bakker, M.L., Nikolic, S., Burrows, A.S., and Alvear, G.R.F., ISACONVERTTM—Continuous converting of nickel/PGM mattes, J. Southern African Inst. Min. Metall., 2011, vol. 111, no. 10, pp. 285–294.
Errington, B., Arthur, P., Wang, J., Dong, Y., The ISA-YMG lead smelting process, Proc. Int. Symp. on Lead and Zinc Processing, Osaka, 2005, pp. 943–960.
Hughes, S., Reuter, M.A., Baxter, R., and Kaye, A., AUSMELT technology for lead and zinc processing, Proc. Lead and Zinc 2008, South African Institute of Mining and Metallurgy (SAIMM), South Africa, pp. 147–162.
Rusakov, M.R., Depletion of slag melts by purging with reducing gases, Tsvet. Met., 1985, no. 3, pp. 40–42.
Komkov, A.A., Baranova, N.V., and Bystrov, V.P., Investigation of reducing depletion of highly oxidized slags under bubbling conditions, Tsvet. Met., 1994, no. 12, pp. 26–30.
Fomichev, V.B., Knyazev, M.V., and Ryumin, A.A., and Tsemekhman, L.Sh., Investigation of slags depletion by purging them with gas mixtures at different partial pressure of oxygen, Tsvet. Met., 2002, no. 9, pp. 32–36.
Komkov, A.A. and Kamkin, R.I., Behavior of copper and impurities when purging copper-smelting slags with CO–CO2 gas mixture, Tsvet. Met., 2011, no. 6, pp. 26–31.
Vusikhis, A.S., Leont’ev, L.I., Chentsov, V.P., Kudinov, D.Z., and Selivanov, E.N., Formation of metallic phase by passing gaseous reducing agent through multicomponent oxide melt. Part 1. Theoretical principles, Steel Transl., 2016, vol. 46, pp. 629–632. https://doi.org/10.3103/S096709121609014X
Vusikhis, A.S., Dmitriev, A.N., Leont’ev, L.I., and Shavrin, S.V., Kinetics of metal oxides reduction from the melt by gas-reducing agent in bubbled layer, Materialovedenie, 2002, no. 10, pp. 30–34.
Vatolin, N.A., Moiseev, G.K., and Trusov, B.G., Termodinamicheskoe modelirovanie v vysokotemperaturnykh neorganicheskikh sistemakh (Thermodynamic Modeling in High-Temperature Inorganic Systems), Moscow: Metallurgiya, 1994.
Boronenkov, V., Zinigrad, M., Leontiev, L., Pastukhov, V.E., Shalimov, M., and Shanchurov, S., Phase Interaction in the Metal–Oxide Melts–Gas System: The Modeling of Structure, Properties and Processes, Engineering Materials, Berlin: Springer, 2012. https://doi.org/10.1007/978-3-642-22377-8
Dmitriev, A.N., Vusikhis, A.S., Sitnikov, V.A., Leontiev, L.I., and Kudinov, D.Z., Thermodynamic modeling of iron oxide reduction by hydrogen from the B2O3–CaO–FeO melt in bubbled layer, Israel J. Chem., 2007, vol. 47, nos. 3–4, pp. 299–302. https://doi.org/10.1560/IJC.47.3-4.299
Vusikhis, A.S., Leont’ev, L.I., Kudinov, D.Z., and Selivanov, E.N., Thermodynamic modeling of nickel and iron reduction from multicomponent silicate melt in bubbling process. Report 1. Reducing agent—A mixture of CO–CO2, Izv. Vyssh. Uchebn. Zaved., Chern. Metall., 2018, vol. 61, no. 9, pp. 731–736. https://doi.org/10.17073/0368-0797-2019-9-731-736
Funding
The work was supported by the Russian Foundation for Basic Research, project no. 18-29-24093 MK.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by A. Muravev
About this article
Cite this article
Vusikhis, A.S., Leont’ev, L.I. & Selivanov, E.N. Thermodynamic Modeling of Iron and Zinc Reduction from B2O3–CaO–Fe2O3–ZnO Melt by СО–СО2 and Н2–Н2О Mixtures. Steel Transl. 52, 308–316 (2022). https://doi.org/10.3103/S0967091222030172
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0967091222030172