Abstract
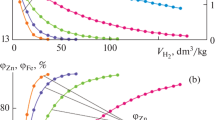
Significance of the research on the metal recovery from the oxide melts is primarily associated with pyrometallurgical treatment of ferrous and non-ferrous metal ores. The main task of the oxidized nickel ore treatment process is to increase the extraction of the valuable metals with the required (10–20%) nickel content in the ferronickel and the minimal amount of admixtures. According to thermodynamic simulation methods, we evaluate the indicators obtained at iron and nickel recovery from the oxide melt. We performed two series of calculations. In the first one, we varied the working body composition against the amount of iron and nickel oxides at the constant CFeO/CNiO ratio equal to 10. In the second one, at the constant CNiO content equal to 1.8%, we varied the CFeO value for the CFeO/CNiO ratios from 10 to 20. The dosed increase of the CO amount in the working body made it possible to trace the compositions changes in the oxide (CMеO) and the metal (CMе) melts, as well as the degrees of nickel (φNi) and iron (φFe) transition to the metal state. We present the CNiO, φNi = f(C0, VCO) correlation dependencies in the form of the second order polynomials. The φNi and the φFe indicators are changed with the introduced reducing agent amount, but depend insufficiently on the initial condensed phase composition. Those are the element contents in the initial melt and the amount of the introduced reducing agent that affect the composition of the formed Fe–Ni alloy. High (65–90%) nickel content is the specific alloys feature. The φNi value of about 98% was achieved at the introduced CO amount of about 80 m3 per melt ton. In that case, the iron recovery degree is within 5%. At the CFeO/CNiO ratio equal to 10, the nickel content in the alloy is in fact independent of its oxide content in the initial ore melt and is close to 65%. A CFeO/CNiO ratio increase from 10 to 20 results in the CNi decrease from 68.5 to 52.9%, respectively. The data obtained are significant for substantiation of the technology of treatment the low-quality oxidized nickel ores aimed at release of the required ferronickel composition.


Similar content being viewed by others
REFERENCES
Leont’ev, L.I., Vatolin, N.A., Shavrin, S.V., and Shumakov, N.S., Pirometallurgicheskaya pererabotka kompleksnykh rud (Pyrometallurgical Processing of Complex Ores), Moscow: Metallurgiya, 1997.
Bugel’skii, Yu.Yu., Vitovskaya, I.V., and Nikitina, A.P., Ekzogennye rudoobrazuyushchie sistemy kory vyvetrivaniya (Exogenous Ore-Forming Systems of Weathering Crust), Moscow: Nauka, 1990.
Bulakh, A.G., Krivovichev, V.G., and Zolotarev, A.A., Obshchaya mineralogiya (General Mineralogy), Moscow: Akademiya, 2008.
Selivanov, E.N., Sergeeva, S.V., Udoeva, L.Yu., and Pankratov, A.A., Dis tribution of nickel by phase components of oxidized nickel ore of the Serovskoye deposit, Obogashch. Rud, 2011, no. 5, pp. 46–50.
Sorokin, E.M., Astakhova, Yu.M., Bystrov, I.G., Ivanova, M.V., et al., Mineralogical and technological features of iron ores of the Serovskoe deposit, Vestn. Inst. Geol., Komi Nauchn. Tsentra, Ural. Otd., Ross. Akad. Nauk, 2015, no. 1, pp. 18–23.
Pakhomov, R.A. and Starykh, R.V., Preliminary reduction of oxidized nickel ores, Russ. Metall. (Engl. Transl.), 2014, vol. 2014, no. 11, pp. 853–860.
Selivanov, E.N. and Sergeeva, S.V., Prospects for the ferronickel produc tion development from the Urals oxidized nickel ores, KnE Mater. Sci., 2019, vol. 5, no. 1, pp. 77–91. https://doi.org/10.18502/kms.v5i1.3954
Chen, J. and Hayes, P.C., Mechanisms and kinetics of reduction of solid NiO in CO/CO2 and CO/Ar gas mixtures, Metall. Mater. Trans. B, 2019, vol. 50, no. 6, pp. 2623–2635. https://doi.org/10.1007/s11663-019-01662-5
Antola, O., Holappa, L., and Paschen, P., Nickel ore reduction by hydrogen and carbon monoxide containing gases, Miner. Process. Extr. Metall. Rev., 1995, vol. 15, nos. 1–4, pp. 169–179. https://doi.org/10.1080/08827509508914195
Liu, M., Liu, X., Guo, E., Chen, P., and Yuan, Q., Novel process of ferronickel nugget production from nickel laterite by semi-molten state reduction, ISIJ Int., 2014, vol. 54, no. 8, pp. 1749–1754. https://doi.org/10.2355/isijinternational.54.1749
Wang, X., Sun, T., Chen, C., and Hu, T., Current studies of treating processes for nickel laterite ores, Proc. 2nd Int. Conf. on Mechatronics Engineering and Information Technology (ICMEIT 2017), Dordrecht: Atlantis Press, 2017, pp. 139–152.
Elliott, R., Pickles, C.A., and Forster, J., Thermodynamics of the reduction roasting of nickeliferous laterite ores, J. Miner. Mater. Charact. Eng., 2016, vol. 4, pp. 320–346. https://doi.org/10.4236/jmmce.2016.46028
Pickles, C.A., Forster, J., and Elliott, R., Thermodynamic analysis of the carbothermic reduction roasting of a nickeliferous limonitic laterite ore, Miner. Eng., 2014, vol. 65, pp. 33–40. https://doi.org/10.1016/j.mineng.2014.05.006
Rhamdhani, M.A., Hayes, P.C., and Jak, E., Nickel laterite, Part 2—Thermodynamic analysis of phase transformations occurring during reduction roasting, Trans. Inst. Min. Metall., Sect. C, 2009, vol. 118, pp. 146–155. https://doi.org/10.1179/174328509X431409
Luoma, R., A thermodynamic analysis of the system Fe–Ni–O, CALPHAD: Comput. Coupling Phase Diagrams Thermochem., 1995, vol. 19, no. 3, pp. 279–295. https://doi.org/10.1016/0364-5916(95)00026-B
Chen, S., Guo, S., Jiang, L., Xu, Y., and Ding, W., Thermodynamic of selective reduction of laterite ore by reducing gases, Trans. Nonferrous Met. Soc. China, 2015, vol. 25, no. 9, pp. 3133–3138. https://doi.org/10.1016/S1003-6326(15)63943-7
Prostakova, V., Chen, J., Jak, E., and Decterov, S.A., Experimental study and thermodynamic modeling of the MgO–NiO–SiO2 system, J. Chem. Thermodyn., 2013, vol. 62, pp. 43–55. https://doi.org/10.1016/j.jct.2013.02.019
Prostakova, V., Chen, J., Jak, E., and Decterov, S.A., Experimental study and thermodynamic optimization of the CaO–NiO, MgO–NiO and NiO–SiO2 systems, CALPHAD: Comput. Coupling Phase Diagrams Thermochem., 2012, vol. 37, pp. 1–10. https://doi.org/10.1016/j.calphad.2011.12.009
Vusikhis, A.S., Dmitriev, A.N., Kudinov, D.Z., and Leontiev, L.I., The study of liquid and gas phases interaction during the reduction of metal oxides from the melts by gas reductant in bubbled layer, Proc. Third Int. Conf. on Mathematical Modeling and Computer Simulation of Materials Technologies (MMT-2004), Ariel, 2004, pp. 72–77.
Dmitriev, A., Leontiev, L, Vusikhis, A., and Kudinov, D., Liquid and gas interaction during reduction in bubbled layer, Proc. European Metallurgical Conf. Emc2005, September 18–21, 2005, Dresden, 2005, vol. 3, pp. 1349–1358.
Funding
The work is performed according to the State Assignment of the Institute of Metallurgy, Ural Branch, RAS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest.
Additional information
Translated by I. Dikhter
About this article
Cite this article
Vusikhis, A.S., Leont’ev, L.I., Selivanov, E.N. et al. Thermodynamic Simulation of Iron and Nickel Recovery from Oxide Melts. Steel Transl. 51, 163–167 (2021). https://doi.org/10.3103/S0967091221030128
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0967091221030128