Abstract
Data on the analysis of the chemical composition and physicochemical properties of native low-metamorphosed coal at the initial moment of its contact with the air are presented. Diffuse reflectance IR spectroscopy, EPR spectroscopy, NMR spectroscopy, gas chromatography, chemical analysis of oxygen-containing groups, and the determination of the specific surface area and wettability of the contacting surface were used to identify changes in the organic matter of coal. The dynamics of changes in the numbers of paramagnetic centers and functional groups showed that the most intense transformations in the surface layer occurred in the first day of coal exposure to air. Next, oxidation at room temperature proceeded in a periodic mode of the accumulation and consumption of radicals and functional O groups. After four days, the process of low-temperature oxidation passed from the accessible outer surface into the diffusion region of the porous space of coal and gradually slowed down.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In coal mining, the process of oxidation starts already at the stage of exposing the seam surface and leads to changes in the physicochemical properties of the near-boundary zone, namely, the chemical composition of surface functional groups, wettability, porosity, fracturing, etc. The intense development of oxidative radical reactions without the possibility of heat removal can lead to the spontaneous combustion of coal both at the stage of mining and in the course of coal transportation, storage, and primary processing. The largest fraction of fires with hard coals belongs to the least metamorphosed long-flame coals, which have the most developed pore surface areas.
Numerous works of Soviet, Russian, and foreign scientists were devoted to the problems of spontaneous combustion, the elucidation of the mechanisms of oxidation by various forms of oxygen, and changes in the composition and properties of coals under oxidative action, among which the most famous are the works of B.F. Meffert [1], G.L. Stadnikov [2], V.S. Krym [3], B.V. Tronov [4], N.M. Karavaev [5], I.I. Ammosov and I.V. Eremin [6], V.S. Veselovskii [7], A.I. Khrisanfova et al. [8], T.A. Kukharenko [9], V.I. Saranchuk [10], V.A. Proskuryakov and A.N. Chistyakov [11], A.I. Kamneva and I.V. Aleksandrov [12], L.F. Butuzova and coauthors [13], M.L. Ulanovskii [14], and D.V. Miroshnichenko and Yu.S. Kaftan [15]. The obtained knowledge was summarized in numerous reviews of methods for assessing the oxidation and spontaneous combustion of coals [14, 16–22].
In general terms, with consideration for many proposed theories, the mechanism of oxidation is formulated as follows: At the first stage, oxygen molecules are adsorbed on the active sites of the coal surface with the formation of carbon–oxygen complexes, which are converted into peroxide and hydroperoxide compounds upon further oxidation. Due to instability, these compounds decompose with the formation of radicals, gaseous products (H2O, CO2, and CO) and oxygen-containing functional groups. The process of oxidation has a radical-chain character, and the number of oxygen-containing groups (hydroxyl, carboxyl, and carbonyl) changes periodically [10, 13]. Under certain conditions (the influence of temperature, humidity, fractional composition, contact time, etc.), the course of the process can accelerate and spread from the surface into the depth of a coal particle or layer.
It is very problematic to track and evaluate these processes in a mine or in a quarry. To accelerate slow heterogeneous oxidative reactions under laboratory conditions, thermal stimulation of coal powder oxidation at temperatures of 70–250°C is often used [10, 13]. At the same time, with an increase in the rate of the process, information on the primary transformations in the organic matter of coal (OMC) upon contact with oxygen is lost. There is no induction period in the kinetic curves [13]. The available information on changes in the technological properties of coals upon oxidation (ash content, moisture content, calorific value, and caking capacity) [10, 15], which forms the basis of technological regulations for storage in warehouses, already takes into account deep transformations in the volume of coal matter. At the same time, the first changes in the surface layer of coal particles (or layers) do not have a noticeable effect on the integral technological characteristics of coal.
In addition, the interpretation and study of the initial stages of oxidation are complicated by the lack of reliable data on the priority of the initial sample, which excludes the possibility of preliminary contact of the samples with air. The use of methods for packing the test material in sealed bags at the site of sampling, waxing, separating the contacted layer in the laboratory, grinding, preparing, and storing the sample with an inert gas purge does not exclude the possibility of coal contact with air at any stages and subsequently using surface-oxidized coal for kinetic studies.
Methods for the sampling, delivery, and cutting of coal samples without air contact for the evaluation of changes in OMC in the first moments of interaction of the exposed active coal surface with oxygen have been developed at the Federal Research Center of Coal and Coal Chemistry, Siberian Branch, Russian Academy of Sciences.
The purpose of this work was to evaluate changes in the composition of OMC upon initial contact with air using sampling and sample cutting methods without air contact.
EXPERIMENTAL
Coal of grade D (long-flame coal) from a promising deposit in the Republic of Khakassia was used as a test sample for studying the initial stage of oxidation. Table 1 summarizes the chemical and technological characteristics of this coal. The interest in long-flame coal from this deposit in the Republic of Khakassia is due to its anomalously high sorption capacity (SBET = 25–50 m2/g) (Table 1), as compared to that of low-metamorphosed coals from other basins. Because of this, it is characterized by an increased tendency to spontaneous combustion in the course of mining, storage, and transportation.
The sample of coal was taken from the freshly exposed surface of a coal seam in the form of large pieces (150–200 mm). The samples were placed in a hermetically sealed plastic container, which was filled with an inert gas and evacuated. Thus, the minimum contact of coal with atmospheric oxygen was ensured and an uncontrolled oxidation of the bulk of the test sample was excluded. Upon delivery to the laboratory, the container with coal was placed through a receiving airlock into a glove box filled with an inert gas (nitrogen of high purity, TU [Technical Specifications] 2114-003-05758954-2007). The presence of oxygen in the box and receiving locks was monitored by an oxygen sensor (an ELAN plus gas analyzer, EKO-INTEKH (Russia); measurement accuracy, 0.005%). The coal was unpacked and all preparatory operations for the analysis of native coal (chipping off the outer surface of large pieces to remove the partially oxidized coal layer, grinding, sieving according to particle sizes, weighing of coal samples, filling of test tubes, ampoules, and cuvettes for further research, pelletization of coal powder, etc.) were carried out in a sealed glove box in a pure inert gas atmosphere. External and excess moisture was removed from native coal by evacuation in an inert atmosphere in the receiving airlock of the glove box.
All methods of analysis (unless otherwise indicated) were performed using a fine fraction of coal with a particle size of <0.1 mm.
The ampoules with samples were opened in the analyzer of a spectrometer or immediately before the analysis. It was assumed that the pores filled with an inert gas and, accordingly, active sites on the coal surface occurred in an inactivated state during the short period of time (no longer than 1 min) when coal was exposed to air between opening the airtight container filled with nitrogen and the beginning of the analysis.
Oxygen-containing groups (OH + COOH) were analyzed by a pH-metric method (a 150MI pH meter, Russia) using ion exchange with sodium hydroxide.
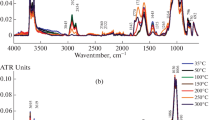
The IR spectra were recorded on a Lyumeks Infralyum FT-08 Fourier transform spectrometer with a PIKE Easydiff diffuse reflectance attachment in a range of 400–4000 cm–1. The IR spectral parameter Ko (oxidation index) was determined from a ratio of the total intensity (the sum of integrated optical densities (D)) of the absorption bands of carboxyl (1710 cm–1) and phenolic + ether (1260 cm–1) groups to the sum of D of aliphatic CHx (2920 cm–1) and aromatic CH (3040 cm–1) groups:
The electron paramagnetic resonance (EPR) spectra were recorded on a Bruker EMX micro 6/1 EPR spectrometer in a rectangular resonator at room temperature in an atmosphere of nitrogen. The number of paramagnetic centers (PMCs) was calculated by comparison with a reference standard (Mn2+ ions in MgO). The main settings of the instrument for recording the spectrum were as follows: magnetic field sweep, 100 G; microwave radiation frequency, ≈9.85 GHz; microwave generator power, 1.85 mW; modulation frequency, 100 kHz; modulation amplitude, 1 G; time constant, 40.96 ms; conversion time, 15 ms; and scan time, 60 s. The spectra were recorded and analyzed using the WinEPR software package. The EPR spectra were subjected to integrated decomposition using the Origin 8 software.
The high-resolution 13C solid-state NMR spectra were recorded on a Bruker Avance III 300 W instrument at 75 MHz using a standard cross-polarization magic angle spinning (CPMAS) technique. To obtain quantitative data, the spectra were simulated using the Dmfit software. Ranges corresponding to the resonant absorption of the following groups of carbon atoms (ppm) were identified in the spectra: (187–171), carbon atoms of carboxyl groups and their derivatives (COO–); (171–148), carbon atoms of aromatic systems associated with an oxygen atom (СarО); (148–129), carbon atoms of condensed aromatic systems (Сar); (129–93), carbon atoms of aromatic systems with an unsubstituted hydrogen atom (CHar); (93–67), carbon atoms of aliphatic structures associated with an oxygen atom (С–О–С); (67–51), carbon atoms of methyl groups associated with an oxygen atom (O–CH3); (51–25), carbon atoms of methylene fragments (CH2); and (25–0), carbon atoms of methyl groups (CH3). Based on an analysis of the spectra, the values of the normalized integral intensities of the main types of carbon structures were determined [23]. The degree of aromaticity of coals was calculated from the formula fa = (СarО + Сar + СНar)/100.
To determine the contact angle of the coal surface, a sample was prepared by pressing powdered coal under a pressure of 700 MPa into a cylindrical briquette 10 mm in diameter and 5 mm high. The briquette was fixed on a glass slide and leveled horizontally, and a droplet of liquid was applied to the test surface through a capillary. The equilibrium shape of the droplet and the contact angle of the coal surface were recorded using a microscope equipped with a video camera [24]. To ensure the reproducibility of the results, at least five coal samples were used, and the droplets were repeatedly applied. The relative error of determination for different methods of surface preparation was 5–10%. Briquettes of native coal obtained in an inert atmosphere immediately after the removal from the box and kept for a certain time in contact with air were used as reference samples.
The chromatographic analysis of the gaseous products of coal oxidation was performed using a Khromatek–Gazokhrom 2000 gas chromatograph. Gas samples (1 cm3) were taken at regular intervals from sealed vessels (100 mL) filled with air and containing native coal samples (5 g) with a particle size of 1–3 mm prepared in an inert atmosphere. The gas sample was injected into the sampling valve of the analyzer to separate the mixture on chromatographic columns. The spectra were processed using the Khromatek-Analitik software.
The samples were analyzed by all of the above methods of analysis for the first seven days when the coal was exposed to air at room temperature and humidity close to standard conditions.
RESULTS AND DISCUSSION
Among many modern physicochemical methods used for analyzing the composition and structure of coals, EPR spectroscopy is characterized by wide possibilities for studying various types of interactions at the initial stage, including low-temperature coal oxidation. The analyzed parameters are the line width and shape of the EPR spectrum, the average signal intensity, the spectroscopic splitting factor (g-factor), and the calculated number of paramagnetic centers (PMCs).
The EPR spectrum of native long-flame coal has a smooth profile consisting of a superposition of signals from several types of radicals. The smooth shape of the profile line indicates the presence of stable radicals in the coal structure [25]. The spectrum has a Lorentz shape characterized by a g-factor of ~2.0031, the line width ∆Н = 3.97 G, and the number of PMCs N = 4.40 × 1019 spin/g (Table 2). The g-factor value is related to the surrounding of the radical with unpaired electrons, and it can be used to determine the type of radical structures. The initial EPR spectrum of coal is integrated into two Lorentzian components, which make it possible to judge the existence of at least two types of PMCs with g = 2.0050 (electrons are localized on the oxygen atom) and with g = 2.0029 (electrons are localized on the carbon atom) [13, 25, 26]. The number of PMCs of the second type exceeds the number of PMCs of the first type by an order of magnitude (Fig. 1), and this fact indicates the dominant contribution of stable hydrocarbon radicals to the chemical composition of the surface of the native low-metamorphosed coal of grade D.
When the native coal entered an oxygen-containing atmosphere, the processes of oxygen sorption by the coal surface and the development of radical-chain oxidation reactions came into play. This was reflected in a significant decrease in the specific surface area determined by nitrogen sorption (SBET) (Fig. 2). Apparently, this was facilitated by the blocking of adsorption centers on the inner walls of the pores by physically adsorbed oxygen and by steric hindrances created by chemisorption products, surface oxygen-containing groups, to the sorbate gas (nitrogen). According to EPR-spectroscopic data, after the first day of coal D exposure to air, the number of PMCs intensely increased due to a more than threefold increase in the concentration of oxygen-containing radicals (g1) and a twofold excess in the number of hydrocarbon radicals (g2). An increase in the number of PMCs affected an increase in the intensity and a broadening of the line in the EPR spectrum (Fig. 1, Table 2).
The increase in the amount of PMCs corresponding to CH and CH2 radicals (g2) at the early stage of coal contact with air was probably associated with the involvement of the hydrocarbon fragments of OMC in chain oxidation reactions. According to IR-spectroscopic data, a decrease in the relative concentrations of aliphatic CHn (2920 and 1380 cm–1) and, to a lesser extent, aromatic CH (3040 and 820 cm–1) groups was detected in the first day of oxidation (Fig. 3). The rapid growth of oxygen-containing radicals (g1) can be due to the activation of OH and C–O groups, as indicated by a decrease in the intensity of absorption of phenolic (3400 cm–1) and ether (1260 cm–1) groups (Fig. 3, Table 2 ). The accumulation of carbonyl (1650 cm–1) and carboxyl (1720 cm–1) groups resulted from oxidative transformations at the initial stage.
After four days, the rate of oxidation of the coal surface slowed down (Figs. 1–3). The amounts of PMCs corresponding to oxygen-containing (g1) and hydrocarbon (g2) radicals changed insignificantly (Fig. 1a), and this can be due to both the formation of new radicals of alkyl and oxygen groups and their involvement in oxidation reactions. Changes in the g-factor values indicate that new radicals structurally different from the radicals that originally existed in coal were formed in the process of low-temperature oxidation of coal. A decrease in the g-factor values from 2.0050 to 2.0047 (g1) or from 2.0029 to 2.0027 (g2) (Fig. 1b) can be due to a decrease in the number of radicals with electron localization on the oxygen atom (for example, phenoxy or alkyl ether radicals) and a relative increase in the number of stable hydrocarbon radicals [25, 26]. Changes in the chemical composition of the coal surface at this stage were mainly associated with the mutual transformations of peripheral functional groups (Fig. 3).
The process stabilized after seven days (Figs. 1–3). The concentration of PMCs, which characterize electrons localized on oxygen atoms (g1), remained near a constant level; the number of PMCs corresponding to hydrocarbon radicals (g2) decreased (Fig. 1a). This became possible due to the simultaneous formation of new radicals and their recombination and decay upon the formation of peripheral functional groups and gaseous products and also due to the transition of the process from the surface of particles to the region of the porous space of coal (slow diffusion stage). The decrease in the specific surface area (SBET), which was noted at the initial stage of the interaction of coal with atmospheric oxygen, significantly slowed down at this stage (Fig. 2).
The most effective way to control the development of the oxidation process is to analyze changes in the composition of gaseous products of the interaction of coal with atmospheric oxygen. The appearance of carbon oxides in the gas atmosphere was detected by gas chromatography already on the first day of the contact of coal with oxygen (Table 3). The concentration of CO and CO2 increased with the duration of oxidation, and the highest intensity of their release corresponded to the fourth day of the exposure of native coal to air. Along with oxygen-containing gases, an increase in the concentration of hydrogen was also noted in the composition of coal oxidation products. The release of CO2, CO, and H2 along with H2O was associated with the decomposition of peroxides, the primary products of coal oxidation [13, 26]. The rate constant of the oxidation process, calculated according a published procedure [27], had a maximum value (0.013 mg g–1 h–1) at the initial stage, and it significantly decreased with increasing the contact time of native coal with air. This means that the initial uptake of oxygen occurred at a high rate, which decreased with time due to the loss of available reactive sites.
Despite the fact that the nuclear magnetic resonance method detects effects in the bulk of coal matter, the results of 13C NMR spectroscopy were no less informative than the data of IR and EPR spectroscopy for assessing the contribution of surface oxidation effects (Table 4). However, in this case, the periodicity of changes in the structural fragments of OMC was inconsistent in time with the EPR- and IR-spectroscopic data (Figs. 2–4). According to the results of 13C NMR spectroscopy, the main changes in OMC upon low-temperature oxidation were associated with a decrease in the relative concentration of ether (OCH3 and C–O–C) groups. In this case, the total number of aliphatic CH3 and alkyl CH2 groups changed insignificantly to indicate the primary transformations of mainly oxygen atoms in the structural units of OMC. Aromatic structural fragments were also resistant to the action of molecular oxygen at ambient temperature, and the degree of aromaticity of coal remained almost unchanged within the duration of the experiment. The number of phenolic groups decreased, while that of carboxyl groups increased. Thus, it can be assumed that peripheral alkyl ether and phenolic groups in the structure of low-metamorphosed long-flame coal are active sites for interaction with oxygen at ambient temperature, and this is consistent with the data of IR spectroscopy and chemical analysis.
Changes in the functional composition of O groups in the surface layer of coal particles affect the wettability of coal, a parameter responsible for the interaction of coal with various liquids, including inhibitors of spontaneous combustion in mines. The method used for determining the wettability of coal surface was very sensitive for detecting and analyzing the results of the initial oxidation. The contact angle θ varied antibatically with the IR-spectral oxidation index (Ko) determined from the ratio between the total intensities of absorption bands due to oxygen-containing and hydrocarbon fragments of OMC (Fig. 4). The wettability increased (the contact angle decreased) with the degree of oxidation of the surface layer of coal at the initial stage of its interaction with air. After four days of coal exposure to air, the contact angle increased again upon stabilization of the process and change in the functional composition (a redistribution of the amounts of OH and COOH groups (Fig. 3)); that is, the surface hydrophobicity increased.
The found changes in the composition of structural fragments of coal in the first few days of its stay in an atmosphere of air did not have a noticeable effect on changes in the integral technological characteristics of coal: the yield of volatile substances, ash content, sintering, calorific value, etc. They cannot be identified by standard methods used for determining the oxidation of coals (petrographic and alkaline methods) because they are limited to a thin surface layer.
With consideration for the concept of the molecular structure of low-metamorphosed coals [16], by analogy with published data [25], it is possible to illustrate changes in the composition of OMC at the initial stage of low-temperature oxidation using a reaction scheme with the participation of initial reactive stable radicals and structural fragments of OMC as an example (Scheme 1).

Scheme 1.
CONCLUSIONS
An analysis of changes in the chemical surface composition and the products of low-temperature oxidation of native coal suggested that the initiation of oxidative radical reactions begins already at the moment of release (opening) of the native surface with the participation of stable radicals present in the structure of coal. Oxidation processes start with the formation of free radicals and their interaction with oxygen sorbed on the coal surface. Because the heterophasic process is limited by the thickness of a diffusion layer, it is likely that chain propagation does not occur for a long time. Free radicals on the exposed coal surface recombine to form gaseous products, stable radicals, and macromolecular oxygen-containing fragments (functional groups). In the case of long-flame coal, the dominant role of reactions with the formation of free radicals loses its significance after 24 h of coal exposure to air. The subsequent reactions are related to mutual transformations of peripheral functional groups. After seven days, the oxidation process slows down and its further development requires the influence of external physicochemical factors that promote the reactivation of stable radicals (humidification, grinding, temperature, UV radiation, etc.).
It is likely that the oxygen-containing radicals of alkyl ether and phenol groups and alkyl radicals are the most reactive species with respect to interactions with oxygen molecules sorbed on the surface of low-metamorphosed long-flame coal.
Coals with increased porosity are characterized by a high rate of development of oxidative processes at the initial stage of low-temperature oxidation due to the free diffusion of an oxidizing agent deep into the porous space.
REFERENCES
Meffert, B.V., O vyvetrivanii mineral’nogo uglya. Tr. Geologicheskogo komiteta. Novaya seriya (On the Weathering of Mineral Coal: Proc. Geological Committee. New Series), St. Petersburg: Tip. M.M. Stasyulevicha, 1910, no. 60.
Stadnikov, G.L., Proiskhozhdenie uglei i nefti (Origin of Coals and Oil), Moscow: Goskhimtekhizdat, 1933.
Krym, V.S., Khimiya tverdogo topliva. Ch. 1. Iskopaemye ugli (Chemistry of Solid Fuel: Part 1. Fossil Coals), Kharkov: Gos. Nauchno-Tekhn. Izd. Ukrainy, 1934.
Tronov, B.V., Okislenie uglei kislorodom vozdukha (Oxidation of Coals by Atmospheric Oxygen), Moscow: Poligrafkniga, 1941.
Karavaev, N.M., Khimiya tverdogo topliva (Chemistry of Solid Fuel), Moscow: Inostrannaya Literatura, 1951.
Ammosov, I.I. and Eremin, I.V., Treshchinovatost’ uglei (Fracturing of Coals), Moscow: Izd. Akad. Nauk SSSR, 1960.
Veselovskii, V.S., Khimicheskaya priroda goryuchikh iskopaemykh (The Chemical Nature of Fossil Fuels), Moscow: Izd. Akad. Nauk SSSR, 1955.
Khrisanfova, A.I., Shubnikov, A.K., Zakharov, A.N., and Gusev, R.P., Ingibitory dlya bor’by s okisleniem i samovozgoraniem iskopaemykh uglei (Inhibitors to Combat the Oxidation and Spontaneous Combustion of Fossil Coals), Moscow: Izd. Akad. Nauk SSSR, 1959.
Kukharenko, T.A., Okislennye v plastakh burye i kamennye ugli (Seam-Oxidized Brown and Black Coals), Moscow: Nedra, 1972.
Saranchuk, V.I., Okislenie i samovozgoranie uglya (Oxidation and Spontaneous Combustion of Coal), Kiev: Naukova Dumka, 1982.
Proskuryakov, V.A. and Chistyakov, A.N., Khim. Tverd. Topl. (Moscow), 1972, no. 2, p. 82.
Kamneva, A.I. and Aleksandrov, I.V., Khim. Tverd. Topl. (Moscow), 1977, no. 4, p. 105.
Kucher, R.V., Kompanets, V.A., and Butuzova, L.F., Struktura iskopaemykh uglei i ikh sposobnost' k okisleniyu (The Structure of Fossil Coals and Their Ability to Oxidize), Kiev: Naukova Dumka, 1980.
Ulanovskii, M.L., Koks Khim., 2012, no. 7, p. 5.
Miroshnichenko, D.V. and Kaftan, Yu.S., Koks Khim., 2017, no. 5, p. 2. https://doi.org/10.3103/S1068364X17050052
van Krevelen, D.W., Coal: Typology, Physics, Chemistry, Constitution, Amsterdam: Elsevier, 1993.
Wang, H., Dlugogorski, B.Z., and Kennedy, E.M., Prog. Energy Combust. Sci., 2003, vol. 29, p. 487.
Taraba, B. and Pavelek, Z., Fuel, 2014, vol. 125, p. 101. https://doi.org/10.1016/j.fuel.2014.02.024
Onifade, M. and Genc, B., Int. J. Mining Sci. Technol., 2020, vol. 30, p. 303. https://doi.org/10.1016/j.ijmst.2020.03.001
Kuznetsov, P.N., Maloletnev, A.S., and Ismagilov, Z.R., Khim. Iteresakh Ustoich. Razvit., 2016, vol. 24, p. 335. https://doi.org/10.15372/KhUR20160308
Epshtein, S.A., Mongush, M.A., and Nesterova, V.G., Gorn. Inf.-Anal. Byull., 2008, no. 12, p. 211.
Semenova, S.A., Patrakov, Yu.F., and Maiorov, A.E., Koks Khim., 2020, no. 5, p. 12.
Rausa, R., Calemma, V., Ghelli, S., and Girardi, E., Fuel, 1989, vol. 68, no. 9, p. 1168. https://doi.org/10.1016/0016-2361(89)90190-7
Patrakov, Yu.F., Semenova, S.A., Kharlampenkova, Yu.A., and Sozinov, S.A., Koks Khim., 2019, no. 12, p. 2. https://doi.org/10.3103/S1068364X19120081
Cai, Ji., Yang, Sh., Zheng, W., Song, W., and Gupta, R., Fuel, 2021, vol. 292, p. 120256. https://doi.org/10.1016/j.fuel.2021.120256
Green, U., Aizenshtat, Z., Ruthstein, Sh., and Cohen, H., Phys. Chem. Chem. Phys., 2012, vol. 14, p. 13046. https://doi.org/10.1039/c2cp41696d
Federal'nye normy i pravila v oblasti promyshlennoi bezopasnosti (Federal Norms and Regulations in the Field of Industrial Safety), Moscow: ZAO NTTs PB, 2013, Ser. 05, no. 38.
ACKNOWLEDGMENTS
We are grateful to Leading Engineers T.A. Papina, A.V. Anikina, and E.S. Nepeina (Institute of Coal, Federal Research Center of Coal and Coal Chemistry, Siberian Branch, Russian Academy of Sciences) and Cand. Sci. (Phys.–Math.) S.A. Sozinov (Center of Collective Use, Federal Research Center of Coal and Coal Chemistry, Siberian Branch, Russian Academy of Sciences) for their assistance in performing analyses by physicochemical methods.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by V. Makhlyarchuk
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Semenova, S.A., Patrakov, Y.F., Yarkova, A.V. et al. Changes in the Properties of Native Low-Metamorphozed Coal in Contact with Air. Solid Fuel Chem. 56, 157–165 (2022). https://doi.org/10.3103/S0361521922030089
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0361521922030089