Abstract
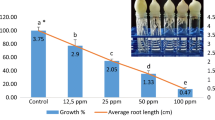
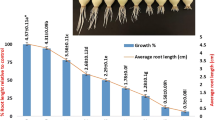
Metaphase-arresting agents amiprophos-methyl (APM), colchicine (COL) and cell cycle-synchronization (CCS) with APM and hydroxyurea (HU) were tested for growth, metaphase index and cytogenetic abnomalities in barley (Hordeum vulgare cv. Bornova-92). Seeds were germinated for 2 days and then seedlings were treated with 8 μM (2.4 mg/L) APM for 2 h or 1.25 mM (0.5 g/L) COL or synchronized (CCS) with 1.25 mM (95 mg/L) hydroxyurea for 18 h and with 4 μM (1.2 mg/L) APM for 2 h. APM and CCS caused metaphase indices 12.57 and 38.82% respectively. COL also arrested metaphase (14.10%) but also resulted in nuclear aberrations (11.15%). After removal of APM and CCS, cells were released to grow and divide. However, COL caused irreversible effects on cell division and growth and meanwhile was shown to be effective for micronucleus formation.
Similar content being viewed by others
References
Altinkut, A. and Gozukirmizi, N., Critical points of fish for the localization of single and low copy sequences in plant chromosomes, Biotechnol. Biotechnol. Equip., 2001, vol. 15, pp. 23–27.
Verhoeven, H.A., Sree, RamuluK., and Dijkhuis, P., A comparison of the effects of various spindle toxins on metaphase arrest and formation of micronuclei in cellsuspension cultures of Nicotiana plumbaginifolia, Planta, 1990, vol. 182, no. 3, pp. 408–414.
Singh, R.J., Plant Cytogenetics, Florida: CRC Press, 2003.
Dolezel, J., Cihalikova, J., Weiserova, J., and Lucretti, S., Cell cycle synchronization in plant root meristems, Meth. Cell Sci, 1999. vol. 21, nos. 2/3, pp. 95–107.
Caperta, A.D., Delgado, M., Ressurreicao, F., et al., Colchicine-induced polyploidization depends on tubulin polymerization in C-metaphase cells, Protoplasma, 2006. vol. 227, nos 2/4, pp. 147–153.
Hantzschel, K.R. and Weber, G., Blockage of mitosis in maize root tips using colchicine-alternatives, Protoplasma, 2010. vol. 241, nos. 1/4, pp. 99–104.
Wu, J.H., Ferguson, A.R., and Murray, B.G., Manipulation of ploidy for kiwifruit breeding: in vitro chromosome doubling in diploid Actinidia chinensis Planch, Plant Cell Tissue Organ Cult., 2011, vol. 106, no. 3, pp. 503–511.
Morejohn, L.C. and Fosket, D.E., Taxol-induced rose microtubule polymerization in vitro and its inhibition by colchicine, J. Cell Biol., 1984, vol. 99, pp. 141–147.
Dhooghe, E., van Laere, K., Eeckhaut, T., et al., Mitotic chromosome doubling of plant tissues in vitro, Plant Cell Tissue Organ Cult., 2011, vol. 104, no. 3, pp. 359–373.
Hansen, N.J.P. and Andersen, S.B., In vitro chromosome doubling potential of colchicine, oryzalin, trifluralin and APM in Brassica napus microspore culture, Euphytica, 1996, vol. 88, no. 2, pp. 159–164.
Hansen, A.L., Gertz, A., Joersbo, M., and Andersen, S.B., Antimicrotubule herbicides for in vitro chromosome doubling in Beta vulgaris L. ovule culture, Euphytica, 1998, vol. 101, no. 2, pp. 231–237.
Jakse, M., Havey, M.J., and Bohanec, B., Chromosome doubling procedures of onion (Allium cepa L.) gynogenic embryos, Plant Cell Rep., 2003, vol. 21, no. 9, pp. 905–910.
Sree, RamuluK., Verhoeven, H.A., and Dijkhuis, P., Mitotic dynamics of micronuclei induced by amiprophosmethyl and prospects for chromosome-mediated gene transfer in plants, Theor. Appl. Genet., 1988, vol. 75, no. 4, pp. 575–584.
Carvalho, C.R., Clarindo, W.R., Praca, M.M., et al., Genome size, base composition and karyotype of Jatropha curcas L., an important biofuel plant, Plant Sci., 2008, vol. 174, no. 6, pp. 613–617.
Young, C.W. and Hodas, S., Hydroxyurea: inhibitory effect on DNA metabolism, Science, 1964, vol. 146, no. 3648, pp. 1172–1174.
Dolezel, J., Cihalikova, J., and Lucretti, S., A high yield procedure for isolation of metaphase chromosomes from root tips of Vicia faba, Planta, 1992, vol. 188, no. 1, pp. 93–98.
Pan, W.H., Houben, A., and Schlegel, R., Highly effective cell synchronization in plant roots by hydroxyurea and amiprophos-methyl or colchicine, Genome, 1993, vol. 36, no. 2, pp. 387–390.
Nonaka, T., Oka, E., Asano, M., et al., Chromosome doubling of Lychnis spp. by in vitro spindle toxin treatment of nodal segments, Sci. Hort., 2011, vol. 129, no. 4, pp. 832–839.
Rodrigues, F.A., Soares, J.D.R., Santos, R.R., et al., Colchicine and amiprophos-methyl (APM) in polyploidy induction in banana plant, Afr. J. Biotech., 2011, vol. 10, no. 62, pp. 13476–13481.
Tchorbadjeva, M.I. and Pantchev, I.Y., DNA methylation and somatic embryogenesis of orchardgrass (Dactylis gloverata L.), Bulg. J. Plant Physiol., 2004. vol. 30, nos. 1/2, pp. 3–13.
Puigderrajols, P., Jofre, A., Mir, G., et al., Developmentally and stress-induced small heat shock proteins in cork oak somatic embryos, J. Exp. Bot., 2002, vol. 53, no. 373, pp. 1445–1452.
Bradford, M.M., A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding, Anal. Biochem., 1976. vol. 72, nos. 1/2, pp. 48–54.
Tkalec, M., Malaric, K., Pavlica, M., et al., Effects of radiofrequency electromagnetic fields on seed germination and root meristematic cells of Allium cepa L., Mutat. Res., 2009, vol. 672, no. 2, pp. 76–81.
Sree, RamuluK., Verhoeven, H.A., and Dijkhuis, P., Mitotic blocking, micronucleation, and chromosome doubling by oryzalin, amiprophos-methyl, and colchicine in potato, Protoplsama, 1991. vol. 160, nos. 2/3, pp. 5–71.
Lee, J.H., Arumuganathan, K., Chung, Y.S., et al., Flow cytometric analysis and chromosome sorting of barley (Hordeum vulgare L.), Mol. Cells, 2000, vol. 10, no. 6, pp. 619–625.
Grosso, V., Farina, A., Gennaro, A., et al., Flow sorting and molecular cytogenetic identification of individual chromosomes of Dasypyrum villosum L. (H. villosa) by a single DNA probe, PLoS One, 2012. vol. 7, no. 11, p. e50151.
Lee, J.H., Arumuganathan, K., Kaeppler, S.M., et al., Cell synchronization and isolation of metaphase chromosomes from maize (Zea mays L.) root tips for flow cytometric analysis and sorting, Genome, 1996, vol. 39, no. 4, pp. 697–703.
Falconer, M.M. and Seagull, R.W., Amiprophosmethyl (APM): a rapid, reversible, anti-microtubule agent for plant cell cultures, Protoplasma, 1987. vol. 163, nos 2/3, pp. 1148–1124.
Mara, C., Dempsey, E., Bell, A., and Barlow, J.W., Synthesis and evaluation of phosphoramidate and phosphorothioamidate analogues of amiprophos methyl as potential antimalarial agents, Bioorg. Med. Chem. Lett., 2011, vol. 21, no. 20, pp. 6180–6183.
Yemets, A.I., Strashnyuk, N.M., and Blume, Y.B., Plant mutants and somatic hybrids with resistance to trifluralin, Cell Biol. Int., 1997, vol. 21, no. 12, pp. 912–914.
Ozheredov, S.P., Yemets, A.I., Brytsun, V.M., et al., Screening of new 2,4 and 2,6-dinitroaniline derivates for phytotoxicity and antimitotic activity, Cytol. Genet., 2009, vol. 43, no. 5, pp. 297–304.
Sree, RamuluK., Verhoeven, H.A., Dijkhuis, P., and Gilissen, L.J.W., Chromosome behaviour and formation of micronuclei after treatment of cell suspension cultures with amiprophos-methyl in various plant species, Plant Sci., 1988, vol. 56, no. 3, pp. 227–237.
Lakshmanan, P.S., Eeckhaut, T., van Huylenbroeck, J., and van Bockstaele, E., Micronucleation by mitosis inhibitors in developing microspores of Spathiphyllum wallissii Regel, Plant Cell Rep., 2013, vol. 32, no. 3, pp. 369–377.
Grzebelus, E. and Adamus, A., Effect of anti-mitotic agents on development and genome doubling of gynogenic onion (Allium cepa L.) embryos, Plant Sci., 2004, vol. 167, no. 3, pp. 569–574.
Pintos, B., Manzanera, J.A., and Bueno, M.A., Antimitotic agents increase the production of doubled-haploid embryos from cork oak anther culture, J. Plant Physiol., 2007, vol. 164, no. 12, pp. 1595–1604.
Kong, W.D., Zhu, Y.G., Liang, Y.C., et al., Uptake of oxytetracycline and its phytotoxicity to alfalfa (Medicago sativa L.), Envrion. Pollut, 2007, vol. 147, no. 1, pp. 187–193.
Yumurtaci, A., Vardar, F., and Unal, M., Inhibition of barley root growth by actinomycin D: effects on mitotic activity, protein content and peroxidase activity, Fresen. Environ. Bull., 2007, vol. 16, no. 8, pp. 917–921.
Jin, C., Chen, Q., Sun, R., et al., Eco-toxic effects of sulfadiazine sodium, sulfamonomethoxine sodium and enrofloxacin on wheat, Chinese cabbage and tomato, Ecotoxicology, 2009, vol. 18, no. 7, pp. 878–885.
Hamal-Mecbur, H., Yilmaz, S., Temel, A., et al., Effects of epirubicin on barley seedligs, Toxicol. Ind. Health, 2014, vol. 30, no. 1, pp. 52–59.
Liu, W., Li, P.J., Qi, X.M., et al., DNA changes in barley (Hordeum vulgare) seedlings induced by cadmium pollution using RAPD analysis, Chemosphere, 2005, vol. 61, no. 2, pp. 158–167.
Anuradha, S. and Rao, S.S.R., Effect of brassinosteroids on salinity stress induced inhibition of seed germination and seedling growth of rice (Oryza sativa L.), Plant Growth Regul., 2001, vol. 33, no. 2, pp. 151–153.
Vakili, N.G., The experimental formation of polyploidy and its effect in the genus Musa, Am. J. Bot., 1967, vol. 54, no. 1, pp. 24–36.
Luckett, D., Colchicine mutagenesis is associated with substantial heritable variation in cotton, Euphytica, 1989. vol. 42, nos. 1/2, pp. 177–182.
Hamill, S.D., Smith, M.K., and Dodd, W.A., In vitro induction of banana autotetraploid by colchicines treatment of micropropagated diploids, Aust. J. Bot., 1992, vol. 40, no. 6, pp. 887–896.
Thao, N.T.P., Ireshino, K., Miyajima, I., et al., Induction of tetraploids in ornamental alocasia through colchicine and oryzalin treatments, Plant Cell Tiss. Org., 2003, vol. 72, no. 1, pp. 19–25.
Wan, Y., Petolino, J.F., and Widholm, J.M., Efficient production of doubled haploid plants through colchicines treatment of anther-derived maize callus, Theor. Appl. Genet., 1989, vol. 77, no. 6, pp. 889–892.
Ranney, T.G., Polyploidy: from evolution to new plant development, Proc. Intl. Plant. Prop. Soc., 2006, vol. 56, pp. 137–142.
Soroka, A.I., Differentiation of haploid and dihaploid rape plants at the cytological and morphological levels, Cytol. Genet., 2013, vol. 47, no. 2, pp. 88–92.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
About this article
Cite this article
Temel, A., Gozukirmizi, N. Cytotoxic effects of metaphase-arresting methods in barley. Cytol. Genet. 49, 382–387 (2015). https://doi.org/10.3103/S0095452715060109
Received:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0095452715060109