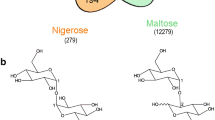
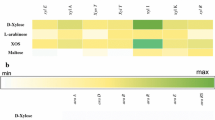
Abstract
α-Glucans from bacterial exo-polysaccharides or diet, e.g., resistant starch, legumes and honey are abundant in the human gut and fermentation of resistant fractions of these α-glucans by probiotic lactobacilli and bifidobacteria impacts human health positively. The ability to degrade polymeric α-glucans is confined to few strains encoding extracellular amylolytic activities of glycoside hydrolase (GH) family 13. Debranching pullulanases of the subfamily GH13_14 are the most common extracellular GH13 enzymes in lactobacilli, whereas corresponding enzymes are mainly α-amylases and amylopullulanases in bifidobacteria. Extracellular GH13 enzymes from both genera are frequently modular and possess starch binding domains, which are important for efficient catalysis and possibly to mediate attachment of cells to starch granules. α-1,6-Linked glucans, e.g., isomalto-oligosaccharides are potential prebiotics. The enzymes targeting these glucans are the most abundant intracellular GHs in bifidobacteria and lactobacilli. A phosphoenolpyruvate-dependent phosphotransferase system and a GH4 phospho-α-glucosidase are likely involved in metabolism of isomaltose and isomaltulose in probiotic lactobacilli based on transcriptional analysis. This specificity within GH4 is unique for lactobacilli, whereas canonical GH13 31 α-1,6-glucosidases active on longer α-1,6-gluco-oligosaccharides are ubiquitous in bifidobacteria and lactobacilli. Malto-oligosaccharide utilization operons encode more complex, diverse, and less biochemically understood activities in bifidobacteria compared to lactobacilli, where important members have been recently described at the molecular level. This review presents some aspects of α-glucan metabolism in probiotic bacteria and highlights vague issues that merit experimental effort, especially oligosaccharide uptake and the functionally unassigned enzymes, featuring in this important facet of glycan turnover by members of the gut microbiota.
Similar content being viewed by others
Abbreviations
- ABC:
-
ATP-binding cassette
- CAZy:
-
Carbohydrate-Active enZymes database
- CBM:
-
carbohydrate binding module
- GH:
-
glycoside hydrolase
- GI:
-
gastrointestinal tract
- GT:
-
glycosyl transferase family
- IMO:
-
isomaltooligosaccharide
- PTS:
-
phosphotransferase transport system
- RS:
-
resistant starch
References
Abou Hachem M., Andersen J.M., Barrangou R., Møller M.S., Fredslund F., Majumder A., Ejby M., Lahtinen S.J., Jacobsen S., Lo Leggio L., Goh Y.J., Klaenhammer T.R. & Svensson B. 2013. Recent insight into oligosaccharide uptake and metabolism in probiotic bacteria. Biocatal. Biotransform. 31: 226–235.
Andersen J.M., Barrangou R., Abou Hachem M., Lahtinen S., Goh Y.J., Svensson B. & Klaenhammer T.R. 2012. Transcriptional analysis of prebiotic uptake and catabolism by Lactobacillus acidophilus NCFM. PloS One 7: e44409.
Andersen J.M., Barrangou R., Abou Hachem M., Lahtinen S., Goh Y.J., Svensson B. & Klaenhammer T.R. 2013. Transcriptional analysis of oligosaccharide utilization by Bifidobacterium lactis Bl-04. BMC Genomics 14: 312.
Arumugam M., Raes J., Pelletier E., Le Paslier D., Yamada T., Mende D.R., Fernandes G.R., Tap J., Bruls T., Batto J.M., Bertalan M., Borruel N., Casellas F., Fernandez L., Gautier L., Hansen T., Hattori M., Hayashi T., Kleerebezem M., Kurokawa K., Leclerc M., Levenez F., Manichanh C., Nielsen H.B., Nielsen T., Pons N., Poulain J., Qin J., Sicheritz-Ponten T., Tims S., Torrents D., Ugarte E., Zoetendal E.G., Wang J., Guarner F., Pedersen O., de Vos W.M., Brunak S., Dore J., Weissenbach J., Ehrlich S.D. & Bork P. 2011. Enterotypes of the human gut microbiome. Nature 473: 174–180.
Barrangou R., Briczinski E.P., Traeger L.L., Loquasto J.R., Richards M., Horvath P., Coute-Monvoisin A.C., Leyer G., Rendulic S., Steele J.L., Broadbent J.R., Oberg T., Dudley E.G., Schuster S., Romero D.A. & Roberts R.F. 2009. Comparison of the complete genome sequences of Bifidobacterium animalis subsp lactis DSM 10140 and Bl-04. J. Bacteriol. 191: 4144–4151.
Belanger A.E. & Hatfull G.F. 1999. Exponential-phase glycogen recycling is essential for growth of Mycobacterium smegmatis. J. Bacteriol. 181: 6670–6678.
Blazek J. & Gilbert E.P. 2010. Effect of enzymatic hydrolysis on native starch granule structure. Biomacromolecules 11: 3275–3289.
Busuioc M., Mackiewicz K., Buttaro B.A. & Piggot P.J. 2009. Role of intracellular polysaccharide in persistence of Streptococcus mutans. J. Bacteriol. 191: 7315–7322.
Cameron E.A., Maynard M.A., Smith C.J., Smith T.J., Koropatkin N.M. & Martens E.C. 2012. Multidomain carbohydratebinding proteins involved in Bacteroides thetaiotaomicron starch metabolism. J. Biol. Chem. 287: 34614–34625.
Cantarel B.L., Coutinho P.M., Rancurel C., Bernard T., Lombard V. & Henrissat B. 2009. The Carbohydrate-Active EnZymes database (CAZy): an expert resource for Glycogenomics. Nucleic Acids Res. 37: D233–D238.
Cantarel B.L., Lombard V. & Henrissat B. 2012. Complex carbohydrate utilization by the healthy human microbiome. PloS One 7: e28742.
Christiansen C., Abou Hachem M., Janecek S., Viksø-Nielsen A., Blennow A. & Svensson B. 2009. The carbohydrate-binding module family 20 — diversity, structure, and function. FEBS J. 276: 5006–5029.
Clemente J.C., Ursell L.K., Parfrey L.W. & Knight R. 2012. The impact of the gut microbiota on human health: an integrative view. Cell 148: 1258–1270.
de Vrese M. & Schrezenmeir J. 2008. Probiotics, prebiotics, and synbiotics, pp. 1–66. In: Stahl U.D.U.E.B.N.E. (ed.) Food Biotechnology.
Duboc P. & Mollet B. 2001. Applications of exopolysaccharides in the dairy industry. Int. Dairy J. 11: 759–768.
Eckburg P.B., Bik E.M., Bernstein C.N., Purdom E., Dethlefsen L., Sargent M., Gill S.R., Nelson K.E. & Relman D.A. 2005. Diversity of the human intestinal microbial flora. Science 308: 1635–1638.
Eydallin G., Montero M., Almagro G., Sesma M.T., Viale A.M., Munoz F.J., Rahimpour M., Baroja-Fernandez E. & Pozueta-Romer J. 2010. Genome-wide screening of genes whose enhanced expression affects glycogen accumulation in Escherichia coli. DNA Res. 17: 61–71.
Eydallin G., Viale A.M., Moran-Zorzano M.T., Munoz F.J., Montero M., Baroja-Fernandez E. & Pozueta-Romero J. 2007. Genome-wide screening of genes affecting glycogen metabolism in Escherichia coli K-12. FEBS Lett. 581: 2947–2953.
Faith J.J., Guruge J.L., Charbonneau M., Subramanian S., Seedorf H., Goodman A.L., Clemente J.C., Knight R., Heath A.C., Leibel R.L., Rosenbaum M. & Gordon J.I. 2013. The long-term stability of the human gut microbiota. Science 341: 44–53.
Flint H.J., Duncan S.H., Scott K.P. & Louis P. 2007. Interactions and competition within the microbial community of the human colon: links between diet and health. Environ. Microbiol. 9: 1101–1111.
Fredslund F., Abou Hachem M., Larsen R.J., Sørensen P.G., Coutinho P.M., Lo Leggio L. & Svensson B. 2011. Crystal structure of α-galactosidase from Lactobacillus acidophilus NCFM: insight into tetramer formation and substrate binding. J. Mol. Biol. 412: 466–480.
Fuentes-Zaragoza E., Sanchez-Zapata E., Sendra E., Sayas E., Navarro C., Fernandez-Lopez J. & Perez-Alvarez J.A. 2011. Resistant starch as prebiotic: a review. Starch 63: 406–415.
Goffin D., Delzenne N., Blecker C., Hanon E., Deroanne C. & Paquot M. 2011. Will isomalto-oligosaccharides, a well-established functional food in Asia, break through the European and American market? The status of knowledge on these prebiotics. Crit. Rev. Food Sci. Nutr. 51: 394–409.
Goh Y.J. & Klaenhammer T.R. 2013. A functional glycogen biosynthesis pathway in Lactobacillus acidophilus: expression and analysis of the glg operon. Mol. Microbiol. 89: 1187–1200.
Jones S.A., Jorgensen M., Chowdhury F.Z., Rodgers R., Hartline J., Leatham M.P., Struve C., Krogfelt K.A., Cohen P.S. & Conway T. 2008. Glycogen and maltose utilization by Escherichia coli O157: H7 in the mouse intestine. Infect. Immun. 76: 2531–2540.
Kaneko T., Kohmoto T., Kikuchi H., Shiota M., Iino H. & Mitsuoka T. 1994. Effects of isomaltooligosaccharides with different degrees of polymerization on human fecal bifidobacteria. Biosci. Biotechnol. Biochem. 58: 2288–2290.
Knudsen A., van Zanten G.C., Jensen S.L., Forssten S.D., Saarinen M., Lahtinen S.J., Bandsholm O., Svensson B., Jespersen L. & Blennow A. 2013. Comparative fermentation of insoluble carbohydrates in an in vitro human feces model spiked with Lactobacillus acidophilus NCFM. Starch-Stärke 65: 346–353.
Kootte R.S., Vrieze A., Holleman F., Dallinga-Thie G.M., Zoetendal E.G., de Vos W.M., Groen A.K., Hoekstra J.B.L., Stroes E.S. & Nieuwdorp M. 2012. The therapeutic potential of manipulating gut microbiota in obesity and type 2 diabetes mellitus. Diabetes Obes. Metab. 14: 112–120.
Ley R.E., Hamady M., Lozupone C., Turnbaugh P.J., Ramey R.R., Bircher J.S., Schlegel M.L., Tucker T.A., Schrenzel M.D., Knight R. & Gordon J.I. 2008. Evolution of mammals and their gut microbes. Science 320: 1647–1651.
Leyer G.J., Li S., Mubasher M.E., Reifer C. & Ouwehand A.C. 2009. Probiotic effects on cold and influenza-like symptom incidence and duration in children. Pediatrics 124: E172–E179.
Loquasto J.R., Barrangou R., Dudley E.G., Stahl B., Chen C. & Roberts R.F. 2013. Bifidobacterium animalis subsp. lactis ATCC 27673 is a genomically unique strain within its conserved subspecies. Appl. Environ. Microbiol. 79: 6903–6910.
Lozupone C.A., Hamady M., Cantarel B.L., Coutinho P.M., Henrissat B., Gordon J.I. & Knight R. 2008. The convergence of carbohydrate active gene repertoires in human gut microbes. Proc. Natl. Acad. Sci. USA 105: 15076–15081.
McFall-Ngai M., Hadfield M.G., Bosch T.C.G., Carey H.V., Domazet-Loso T., Douglas A.E., Dubilier N., Eberl G., Fukami T., Gilbert S.F., Hentschel U., King N., Kjelleberg S., Knoll A.H., Kremer N., Mazmanian S.K., Metcalf J.L., Nealson K., Pierce N.E., Rawls J.F., Reid A., Ruby E.G., Rumpho M., Sanders J.G., Tautz D. & Wernegreen J.J. 2013. Animals in a bacterial world, a new imperative for the life sciences. Proc. Natl. Acad. Sci. USA 110: 3229–3236.
Møller M.S., Fredslund F., Majumder A., Nakai H., Poulsen J.C.N., Lo Leggio L., Svensson B. & Abou Hachem M. 2012. Enzymology and structure of the GH13_31 glucan 1,6-α-glucosidase that confers isomaltooligosaccharide utilization in the probiotic Lactobacillus acidophilus NCFM. J. Bacteriol. 194: 4249–4259.
Morgan X.C., Segata N. & Huttenhower C. 2013. Biodiversity and functional genomics in the human microbiome. Trends Genet. 29: 51–58.
Muegge B.D., Kuczynski J., Knights D., Clemente J.C., Gonzalez A., Fontana L., Henrissat B., Knight R. & Gordon J.I. 2011. Diet drives convergence in gut microbiome functions across mammalian phylogeny and within humans. Science 332: 970–974.
Nakai H., Baumann M.J., Petersen B.O., Westphal Y., Schols H., Dilokpimol A., Abou Hachem M., Lahtinen S.J., Duus J.O. & Svensson B. 2009. The maltodextrin transport system and metabolism in Lactobacillus acidophilus NCFM and production of novel α-glucosides through reverse phosphorolysis by maltose phosphorylase. FEBS J. 276: 7353–7365.
Nicholson J.K., Holmes E., Kinross J., Burcelin R., Gibson G., Jia W. & Pettersson S. 2012. Host-gut microbiota metabolic interactions. Science 336: 1262–1267.
Petrova P., Petrov K. & Stoyancheva G. 2013. Starch-modifying enzymes of lactic acid bacteria — structures, properties, and applications. Starch-Stärke 65: 34–47.
Rastall R.A. 2010. Functional oligosaccharides: application and manufacture. Annu. Rev. Food Scie. Technol. 1: 305–339.
Rodriguez-Sanoja R., Ruiz B., Guyot J.P. & Sanchez S. 2005. Starch-binding domain affects catalysis in two Lactobacillus α-amylases. Appl. Environ. Microbiol. 71: 297–302.
Sanders M.E. & Klaenhammer T.R. 2001. Invited review: The scientific basis of Lactobacillus acidophilus NCFM functionality as a probiotic. J. Dairy Sci. 84: 319–331.
Sanz M.L., Gibson G.R. & Rastall R.A. 2005. Influence of disaccharide structure on prebiotic selectivity in vitro. J. Agric. Food Chem. 53: 5192–5199.
Sarbini S.R., Kolida S., Gibson G.R. & Rastall R.A. 2013. In vitro fermentation of commercial α-gluco-oligosaccharide by faecal microbiota from lean and obese human subjects. Br. J. Nutr. 109: 1980–1989.
Scott K.P., Gratz S.W., Sheridan P.O., Flint H.J. & Duncan S.H. 2013. The influence of diet on the gut microbiota. Pharmacol. Res. 69: 52–60.
Sommer F. & Baeckhed F. 2012. The gut microbiota — masters of host development and physiology. Nat. Rev. Microbiol. 11: 227–238.
Stam M.R., Danchin E.G.J., Rancurel C., Coutinho P.M. & Henrissat B. 2006. Dividing the large glycoside hydrolase family 13 into subfamilies: towards improved functional annotations of α-amylase-related proteins. Protein Eng. Des. Sel. 19: 555–562.
Tang M.L.K., Lahtinen S.J. & Boyle R.J. 2010. Probiotics and prebiotics: clinical effects in allergic disease. Curr. Opin. Pediatr. 22: 626–634.
Tester R.F., Karkalas J. & Qi X. 2004. Starch — composition, fine structure and architecture. J. Cereal Sci. 39: 151–165.
Thompson J., Jakubovics N., Abraham B., Hess S. & Pikis A. 2008. The sim operon facilitates the transport and metabolism of sucrose isomers in Lactobacillus casei ATCC 334. J. Bacteriol. 190: 3362–3373.
Vigsnæs L.K., Nakai H., Hemmingsen L., Andersen J.M., Lahtinen S.J., Rasmussen L.E., Abou Hachem M., Petersen B.O., Duus J.O., Meyer A.S., Licht T.R. & Svensson B. 2013. In vitro growth of four individual human gut bacteria on oligosaccharides produced by chemoenzymatic synthesis. Food Funct. 4: 784–793.
Wallace T.C., Guarner F., Madsen K., Cabana M.D., Gibson G., Hentges E. & Sanders M.E. 2011. Human gut microbiota and its relationship to health and disease. Nutr. Rev. 69: 392–403.
Whelan K. 2011. Probiotics and prebiotics in the management of irritable bowel syndrome: a review of recent clinical trials and systematic reviews. Curr. Opin. Clin. Nutr. Metab. Care 14: 581–587.
Yen C.H., Tseng Y.H., Kuo Y.W., Lee M.C. & Chen H.L. 2011. Long-term supplementation of isomalto-oligosaccharides improved colonic microflora profile, bowel function, and blood cholesterol levels in constipated elderly people — a placebocontrolled, diet-controlled trial. Nutrition 27: 445–450.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Møller, M.S., Goh, Y.J., Viborg, A.H. et al. Recent insight in α-glucan metabolism in probiotic bacteria. Biologia 69, 713–721 (2014). https://doi.org/10.2478/s11756-014-0367-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11756-014-0367-7